复合生物墨水中的细胞驱动弹性颗粒填料,用于工程设计和植入稳定的 3D 打印结构
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
由于细胞压实和水凝胶收缩,三维打印的细胞构建体的几何和结构完整性往往会随着时间的推移而退化。本研究介绍了一种新方法,它将生物水凝胶的细胞兼容性和弹性聚合物的机械稳定性优势结合在一起,从而在立体光刻和挤出三维打印过程中保持结构的真实性。这种复合生物墨水是通过将聚马来酸(酐)柠檬酸八亚甲基酯(POMaC)弹性微粒与生物衍生水凝胶(纤维蛋白、甲基丙烯酰明胶(GelMA)和海藻酸)结合而配制的。复合生物墨水增强了三维打印结构的弹性和可塑性,有效缓解了组织压实和肿胀。它具有剪切模量低、交联时间快、极限抗压强度高、抗细胞力和物理操作变形的特点;这归功于弹性颗粒的堆积和应力消散,并通过数学建模得到了证实。人类 iPSC 衍生心脏组织和原始血管的功能组装和稳定性的增强证明了复合生物墨水在组织工程中的实用性。体内植入研究表明,含有POMaC颗粒的构建体对宿主组织应力的恢复能力更强,血管生成和促复原巨噬细胞的浸润也得到了增强。本文章由计算机程序翻译,如有差异,请以英文原文为准。
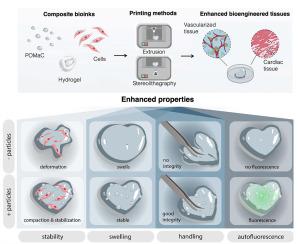
Cell driven elastomeric particle packing in composite bioinks for engineering and implantation of stable 3D printed structures
Geometric and structural integrity often deteriorate in 3D printed cell-laden constructs over time due to cellular compaction and hydrogel shrinkage. This study introduces a new approach that synergizes the advantages of cell compatibility of biological hydrogels and mechanical stability of elastomeric polymers for structure fidelity maintenance upon stereolithography and extrusion 3D printing. Enabling this advance is the composite bioink, formulated by integrating elastomeric microparticles from poly(octamethylene maleate (anhydride) citrate) (POMaC) into biologically derived hydrogels (fibrin, gelatin methacryloyl (GelMA), and alginate). The composite bioink enhanced the elasticity and plasticity of the 3D printed constructs, effectively mitigating tissue compaction and swelling. It exhibited a low shear modulus and a rapid crosslinking time, along with a high ultimate compressive strength and resistance to deformation from cellular forces and physical handling; this was attributed to packing and stress dissipation of elastomeric particles, which was confirmed via mathematical modelling. Enhanced functional assembly and stability of human iPSC-derived cardiac tissues and primary vasculature proved the utility of the composite bioink in tissue engineering. In vivo implantation studies revealed that constructs containing POMaC particles exhibited improved resilience against host tissue stress, enhanced angiogenesis, and infiltration of pro-reparative macrophages.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


