IGFALS 通过稳定 PPAR-γ 抑制肝细胞癌的发展
IF 4.8
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
IGFALS 与胰岛素样生长因子(IGF1 和 IGF2)和 IGF 结合蛋白(IGFBP3 和 IGFBP5)形成稳定的三元复合物,从而延长了 IGF 的半衰期。通过对 90 对临床样本进行免疫组化分析和生物信息学分析,我们观察到 IGFALS 在肝细胞癌组织中的下调,这与患者的不良预后有关。这促使我们探索IGFALS抑制肝细胞癌(HCC)的具体分子作用机制,这可能是治疗HCC的潜在新靶点。体外实验证明,IGFALS能抑制肝癌细胞的增殖、侵袭和迁移,并抑制上皮-间质转化。基因组富集分析(Gene Set Enrichment Analysis,GSEA)显示,IGFALS与PPAR通路的激活呈正相关。Western印迹、免疫荧光共定位和共免疫沉淀试验证实,IGFALS与PPAR-γ结合,并通过去泛素化使其稳定。抑制 PPAR-γ 可逆转 IGFALS 的抗癌作用。此外,我们还发现 IGFALS/PPAR-γ 能上调 HMGCS2 的表达。肿瘤异种移植模型支持了我们的发现。质谱分析和共沉淀实验表明,IGFALS 促进 PPAR-γ 与去泛素化酶 USP9X 结合,从而促进 PPAR-γ 的去泛素化。总之,我们的研究结果表明,IGFALS可通过PPAR-γ/HMGCS2途径抑制肝细胞癌。本文章由计算机程序翻译,如有差异,请以英文原文为准。
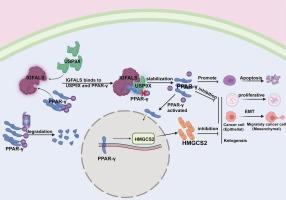
IGFALS suppresses hepatocellular carcinoma progression by stabilizing PPAR-γ
IGFALS forms stable ternary complexes with insulin-like growth factors (IGF1 and IGF2) and IGF-binding proteins (IGFBP3 and IGFBP5), which prolong the half-lives of IGFs. Through immunohistochemical analysis of 90 pairs of clinical samples and bioinformatics analysis, we observed downregulation of IGFALS in hepatocellular carcinoma tissues, which was associated with poor patient prognosis. This prompted us to explore the specific molecular mechanism of action of IGFALS in the inhibition of hepatocellular carcinoma (HCC), which could be a potential new target for the treatment of HCC. In vitro experiments demonstrated that IGFALS inhibits the proliferation, invasion, and migration of hepatocellular carcinoma cells and suppresses epithelial-mesenchymal transition. Gene Set Enrichment Analysis (GSEA) revealed a positive correlation between IGFALS and the activation of the PPAR pathway. Western blotting, immunofluorescence colocalization, and co-immunoprecipitation assays confirmed that IGFALS binds to PPAR-γ and stabilizes it through deubiquitination. Inhibition of PPAR-γ reversed the anticancer effects of IGFALS. Furthermore, we showed that IGFALS/PPAR-γ upregulates the expression of HMGCS2. The tumor xenograft model supported our findings. Mass spectrometry analysis and co-immunoprecipitation assays indicated that IGFALS promotes the binding of PPAR-γ with USP9X, a deubiquitinating enzyme, thereby facilitating the deubiquitination of PPAR-γ. In conclusion, our findings demonstrate that IGFALS can suppress hepatocellular carcinoma via the PPAR-γ/HMGCS2 pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


