从大理石污泥中提取的生态羟基磷灰石增强了对铅的吸附:优化和动力学研究
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究以大理石污泥为原料,通过水热法合成了生态羟基磷灰石(e-HAP),并将其用作去除废水中 Pb2+ 离子的吸附剂。X 射线粉末衍射(XRD)和热场发射扫描电子显微镜(FE-SEM)显示,提高 Ca/P 摩尔比(CPMR)和水热温度(HT)可增加结晶度,而 HT 的影响更为明显。随着 CPMR 的增加,结晶度、晶体长度和峰值强度也随之增加。吸附研究表明,当 HT 为 393 K 时,CPMR 为 0.67,初始 Pb2+ 离子浓度为 100 mg/L,平衡时间为 20 分钟,吸附剂用量为 0.6 g/L。吸附剂的去除率(99.99 %)和最大吸附容量(166.65 mg/g)都非常高。使用伪二阶动力学模型对吸附动力学进行了很好的描述。本文提出了一种从大理石污泥中合成 e-HAP 的简便且经济上可持续的方法,可用于去除废水中的 Pb2+。本文章由计算机程序翻译,如有差异,请以英文原文为准。
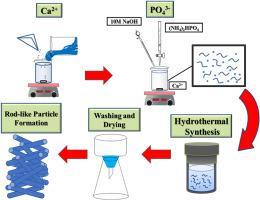
Enhanced lead adsorption by eco-hydroxyapatite derived from marble sludge: Optimization and kinetic study
In this study, eco-hydroxyapatite (e-HAP) was synthesized from marble sludge via a hydrothermal process for utilization as an adsorbent in the removal of Pb2+ ions from wastewater. X-ray powder diffraction (XRD) and thermal field emission scanning electron microscope (FE-SEM) revealed that increasing the Ca/P molar ratio (CPMR) and hydrothermal temperature (HT) increased crystallinity and that the influence of HT was more apparent. An increase in crystallinity, crystal length, and peak intensity was observed following an increase in the CPMR. Adsorption studies show that when the HT was 393 K, the CPMR was 0.67, the initial Pb2+ ion concentration was 100 mg/L, the equilibrium time was 20 min, and the adsorbent dosage was 0.6 g/L. The resulting removal efficiency (99.99 %) and maximum adsorption capacity (166.65 mg/g) are both very high. Adsorption kinetics are well described using a pseudo-second-order kinetic model. This paper presents a facile and economically sustainable approach to the synthesis of e-HAP from marble sludge for use in removing of Pb2+ from wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


