利用 smile's 重排法合成 Metopimazine 的简单而可靠的方法。通过衍生化消除基因毒性杂质
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们开发了一种简单、高效和可扩展的方法来合成多巴胺 D2 受体拮抗剂 Metopimazine。从硝基苯硫代甲磺酰苯胺开始,使用甲酸进行简单的 N-甲酰化反应,然后在碱催化下进行环化反应,最终形成 2-(甲磺酰基)-10H-吩噻嗪。这种方法不涉及传统的过酸氧化反应,而过酸氧化反应会形成多种氧化物,分离起来非常麻烦。事实证明,后一种衍生物在合成标题活性药物成分时非常有效,先进行 N-氯丙基化反应,然后再处理哌啶-4-甲酰胺,这样就能以高产率生成美托咪嗪。硝基芳香族衍生物等潜在的基因毒性杂质通过使用简单的锌粉甲酸还原法有效去除,该方法可有效地将硝基衍生物转化为可溶于水的胺盐酸盐。同样,吩噻嗪衍生物的 N-烷基化也会产生潜在的杂质,如形成 N-烯丙基和丙基桥接二聚体衍生物,这些杂质可通过有效的重结晶去除。该方法已进行了放大,证明在产量方面没有受到任何影响,因此可被视为工业上的现成方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
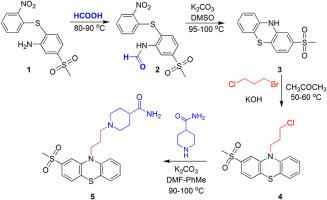
Simple and robust method for the synthesis of Metopimazine by utilising smile’s rearrangement. Elimination of genotoxic impurities via derivatization
A simple, efficient and scalable methodology has been developed for the synthesis of Metopimazine, a dopamine D2–receptor antagonist. Starting from nitrophenylthio substituted methylsulfonyl benzenamine subjected to simple N–formylation using formic acid followed by base–catalysed cyclization resulted the formation of 2–(methylsulfonyl)–10H–phenothiazine. This method does not involve the conventional peracid oxidation that exemplifies the formation of several oxidized species that are tedious to separate. The later derivative is proven effective in the synthesis of title active pharmaceutical ingredient via N–chloroproylation followed by treating piperidine–4–carboxamide resulted in the formation of Metopimazine in high yield. The potential genotoxic impurities such as nitroaromatic derivatives were effectively removed by reduction using simple zinc dust formic acid treatment, which effectually converts nitro derivatives into amine hydrochlorides that are water soluble. Similarly, the N–alkylation of phenothiazine derivative forms potential impurities such as the formation of N–allyl and propyl bridged dimer derivatives that are removed by effective recrystallization. This method has been scaled up and evidenced no compromise with the yield and hence can be considered as industry ready.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


