气相与 BaFeO3-δ 氧化物之间的 16O2 - 18O2 界面交换研究
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在 350-600 °С 的温度范围内,采用氧同位素交换法,在氧分压为 21.3 kPa 的条件下脉冲供应同位素富集混合物 (PIE),对 BaFeO3-δ 氧化物的氧表面交换动力学进行了研究。氧表面交换动力学在两步模型框架内进行了研究,包括两个连续步骤:氧的离解吸附和氧原子融入氧化物晶格。氧异质交换速率(rH)以及解离吸附速率(ra)和氧掺入速率(ri)均已计算出来。研究发现,氧在 BaFeO3-δ 氧化物表面的离解吸附过程是表面交换的速率决定步骤。讨论了描述 "气态氧-BaFeO3-δ 氧化物 "系统中氧交换动力学的适当模型和可能发生的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
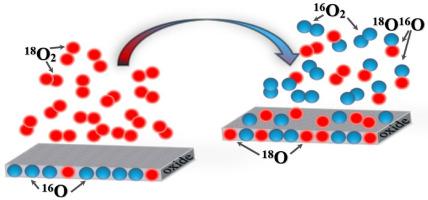
16O2 – 18O2 interface exchange study between gas phase and the BaFeO3–δ oxide
Studies of oxygen surface exchange kinetics for BaFeO3–δ oxide were performed using the oxygen isotope exchange method with pulsed supply of an isotopically enriched mixture (PIE) at the partial oxygen pressure 21.3 kPa in the temperature range of 350–600 °С. Oxygen surface exchange kinetics was considered in the framework of two-step model including two consecutive steps: dissociative adsorption of oxygen and incorporation of oxygen adatoms into the crystal lattice of the oxide. The rates of oxygen heterogeneous exchange (rH), as well as the rates of dissociative adsorption (ra) and oxygen incorporation (ri), have been calculated. The process of oxygen dissociative adsorption at the surface of BaFeO3–δ oxide was found to be the rate-determining step of the surface exchange. The appropriate models describing the oxygen exchange kinetics and possible mechanisms occurring in the system “gaseous oxygen – BaFeO3–δ oxide” were discussed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


