富含淋巴细胞肝癌的临床病理特征和肿瘤免疫微环境
IF 1.5
4区 医学
Q3 PATHOLOGY
引用次数: 0
摘要
富淋巴细胞肝细胞癌(LR-HCC)是一种罕见的HCC变异型,其特点是明显的淋巴细胞浸润,为探索肿瘤免疫微环境(TIME)及其对疾病进展和治疗的潜在影响提供了机会。本研究旨在描述 LR-HCC 的临床病理特征和 TIME 成分,为更有效的治疗策略提供依据。在本研究中,我们在对 136 例既往病例进行全面回顾性分析的同时,还介绍了五例新的 LR-HCC 病例。我们利用免疫组化评估系统地评估了TIME成分和免疫检查点抑制剂(ICI)靶点。我们的研究结果表明,CD3+ T细胞明显多于CD20+ B细胞(1.5:1, P <0.001),CD8+细胞毒性T细胞多于Foxp3+调节性T细胞(2.4:1, P <0.001),这表明免疫格局可能有利于免疫治疗干预。使用 VENTANA SP263 检测法,5 例病例中有 3 例检测到程序性细胞死亡配体 1(PD-L1)表达,这表明患者可能对 ICIs 有反应。对38例病例的汇总分析显示,5年总生存率为73.6%,明显低于之前报道的比率(90%),29.4%的患者术后复发或淋巴结转移。多变量分析发现,肿瘤大小是总生存率的独立预测因素。这些发现强调了TIME特征与了解LR-HCC的相关性,并为靶向和免疫疗法指明了前景广阔的途径,有助于优化这一独特癌症亚型的临床治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
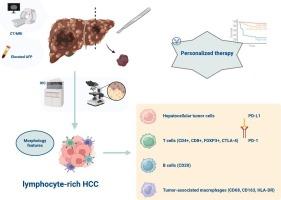
Clinicopathologic features and tumor immune microenvironment of lymphocyte-rich hepatocellular carcinoma
Lymphocyte-rich hepatocellular carcinoma (LR-HCC) is a rare variant of HCC characterized by pronounced lymphoid infiltration, providing an opportunity to explore the tumor immune microenvironment (TIME) and its potential impact on disease progression and therapy. This study aimed to describe the clinicopathological features and TIME components of LR-HCC to inform more effective treatment strategies. In this study, we present five novel cases of LR-HCC alongside a comprehensive retrospective analysis of 136 previously documented cases. Immunohistochemical evaluation was utilized to systematically assess TIME components and immune checkpoint inhibitor (ICI) targets. Our findings demonstrated a significant predominance of CD3+ T cells over CD20+ B cells (1.5:1, P < 0.001) and a higher frequency of CD8+ cytotoxic T cells compared to Foxp3+ regulatory T cells (2.4:1, P < 0.001), indicating an immune landscape potentially favorable for immunotherapeutic interventions. Programmed cell death ligand 1 (PD-L1) expression was detected in three out of five cases using the VENTANA SP263 assay, suggesting potential responsiveness to ICIs. A pooled analysis of 38 cases showed a 5-year overall survival rate of 73.6 %, which is notably lower than previously reported rates (>90 %), with 29.4 % of patients experiencing postoperative recurrence or lymph node metastasis. Multivariate analysis identified tumor size as an independent predictor of overall survival. These findings emphasize the relevance of TIME characteristics in understanding LR-HCC and point to promising avenues for targeted and immune-based therapies, contributing to the optimization of clinical management for this distinct cancer subtype.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.90
自引率
5.00%
发文量
149
审稿时长
26 days
期刊介绍:
A peer-reviewed journal devoted to the publication of articles dealing with traditional morphologic studies using standard diagnostic techniques and stressing clinicopathological correlations and scientific observation of relevance to the daily practice of pathology. Special features include pathologic-radiologic correlations and pathologic-cytologic correlations.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


