光热复合聚脒肟-氧化石墨烯/聚丙烯酰胺水凝胶用于从海水中高效、选择性提取铀
IF 8.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
从海水中提取铀被认为是将彻底改变世界的七大分离技术之一。尽管含有脒肟(AO)基团的吸附剂已显示出良好的效果,但其容量和选择性仍是一项挑战。本研究采用紫外线光引发策略制备了聚脒肟-氧化石墨烯/聚丙烯酰胺(PAO-GO/PAM)光热复合水凝胶。通过丙烯酰胺的自由基聚合在分子水平上形成的三维亲水网络保留了大量的 PAO,有利于铀酰离子(UO22+)迁移到吸附剂内部与 AO 基团螯合。此外,GO 的引入还为水凝胶提供了出色的光热转换能力,使其在暴露于模拟阳光下时温度迅速升高。根据与 AO 基团螯合的 UO22+ 的吸热特性,水凝胶的温度升高必然导致热力学平衡的右移,从而使制备的水凝胶在光照条件下具有较高的吸附能力。吸附实验表明,PAO50-GO1.5/PAM 水凝胶在 pH = 6 时的最大吸附容量为 396.82 mg-g-1,KU/KV 比值提高了 63.4%。经过五个循环后,洗脱率保持在 87.1%。这些结果表明,该吸附剂在大规模提取海水中的铀方面具有广阔的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
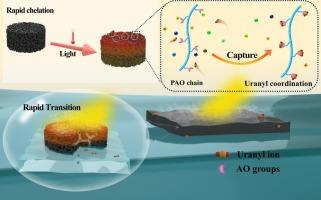
Photothermal composite polyamidoxime-graphene oxide/polyacrylamide hydrogel for efficient and selective uranium extraction from seawater
Uranium extraction from seawater is considered to be one of the seven separation technologies that will revolutionize the world. Although adsorbents containing amidoxime (AO) groups have shown promising results, their capacity and selectivity still pose challenges. In this study, polyamidoxime-graphene oxide/polyacrylamide (PAO-GO/PAM) photothermal composite hydrogels were prepared using an ultraviolet photoinitiation strategy. The three-dimensional (3D) hydrophilic network at the molecular level by radical polymerization of acrylamide retains a large amount of PAO, facilitating the easy migration of uranyl ions (UO22+) to the interior of the adsorbent to chelate with AO groups. In addition, GO was introduced to provide the hydrogel with excellent photothermal conversion ability, causing a rapid temperature increase upon exposure to simulated sunlight. Based on the heat-absorbing properties of UO22+ chelated with AO groups, the temperature increase of the hydrogel inevitably leads to a right-shift of the thermodynamic equilibrium, providing the prepared hydrogel with a high adsorption capacity under light conditions. Adsorption experiments showed that the PAO50-GO1.5/PAM hydrogel achieves a maximum adsorption capacity of 396.82 mg·g−1 at pH = 6 with an improvement of 63.4 % in the KU/KV ratio. After five cycles, the elution rate remained at 87.1 %. These results demonstrate the promising applications of the adsorbent in large-scale uranium extraction from seawater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


