通过装饰金属有机框架材料的结构缺陷捕获痕量苯
IF 37.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
捕获痕量苯是一项重要而具有挑战性的任务。金属有机框架材料是一种很有前景的吸附剂,可吸附多种气体,但其对低浓度苯的吸附能力有限,这一问题仍未得到解决。在此,我们报告了通过用单原子金属中心装饰 MIL-125 的结构缺陷,从而得到 MIL-125-X(X = Mn、Fe、Co、Ni、Cu、Zn;MIL-125,Ti8O8(OH)4(BDC)6,其中 H2BDC 为 1,4-苯二甲酸)来吸附痕量苯的情况。在 298 K 时,MIL-125-Zn 在 1.2 毫巴和 0.12 毫巴条件下的苯吸收率分别为 7.63 毫摩尔/克和 5.33 毫摩尔/克,突破实验证实,即使暴露在潮湿环境中,也能从空气中去除痕量苯(从 5 ppm 到 0.5 ppm)(金属有机框架的去除率高达 111,000 分钟/克)。通过衍射、散射和光谱分析,可以看到苯在低压下与缺陷和开放的 Zn(II)位点结合的情况。这项工作强调了微调孔化学性质对于设计去除空气污染物的吸附剂的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
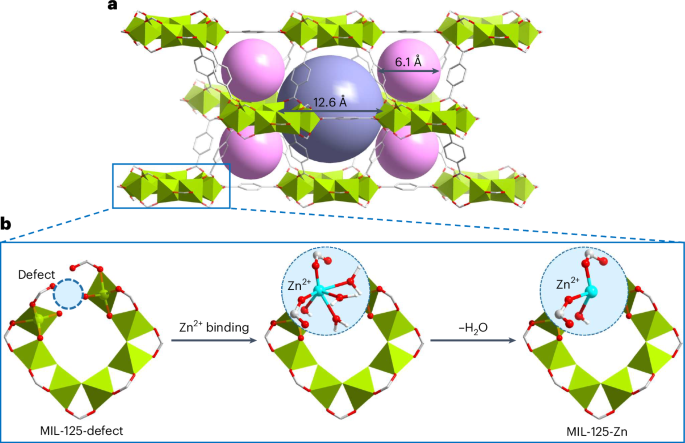
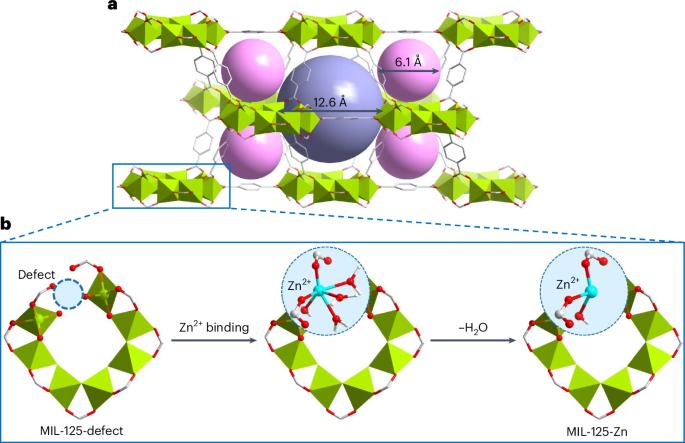
Trace benzene capture by decoration of structural defects in metal–organic framework materials
Capture of trace benzene is an important and challenging task. Metal–organic framework materials are promising sorbents for a variety of gases, but their limited capacity towards benzene at low concentration remains unresolved. Here we report the adsorption of trace benzene by decorating a structural defect in MIL-125-defect with single-atom metal centres to afford MIL-125-X (X = Mn, Fe, Co, Ni, Cu, Zn; MIL-125, Ti8O8(OH)4(BDC)6 where H2BDC is 1,4-benzenedicarboxylic acid). At 298 K, MIL-125-Zn exhibits a benzene uptake of 7.63 mmol g−1 at 1.2 mbar and 5.33 mmol g−1 at 0.12 mbar, and breakthrough experiments confirm the removal of trace benzene (from 5 to <0.5 ppm) from air (up to 111,000 min g−1 of metal–organic framework), even after exposure to moisture. The binding of benzene to the defect and open Zn(II) sites at low pressure has been visualized by diffraction, scattering and spectroscopy. This work highlights the importance of fine-tuning pore chemistry for designing adsorbents for the removal of air pollutants. Benzene is a genotoxic carcinogen with no safe level of exposure. Here, by creating and decorating a structural defect in a metal–organic framework to form MIL-125-Zn, a benzene uptake of 7.63 mmol g–1 at 1.2 mbar is observed due to binding to Zn(II) sites.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Materials
工程技术-材料科学:综合
CiteScore
62.20
自引率
0.70%
发文量
221
审稿时长
3.2 months
期刊介绍:
Nature Materials is a monthly multi-disciplinary journal aimed at bringing together cutting-edge research across the entire spectrum of materials science and engineering. It covers all applied and fundamental aspects of the synthesis/processing, structure/composition, properties, and performance of materials. The journal recognizes that materials research has an increasing impact on classical disciplines such as physics, chemistry, and biology.
Additionally, Nature Materials provides a forum for the development of a common identity among materials scientists and encourages interdisciplinary collaboration. It takes an integrated and balanced approach to all areas of materials research, fostering the exchange of ideas between scientists involved in different disciplines.
Nature Materials is an invaluable resource for scientists in academia and industry who are active in discovering and developing materials and materials-related concepts. It offers engaging and informative papers of exceptional significance and quality, with the aim of influencing the development of society in the future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


