调节与纳米铁粒子结合的绿锈中由层间 SO42 诱导的 SeO42- 反弹,用于地下水修复
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
绿锈(GR)是一种层间含阴离子的铁(II)/铁(III)矿物材料,具有去除水中一系列离子污染物的多功能性。本研究以 SeO42- (Se(VI))为目标污染物,发现铁锈对 Se(VI)的去除过程可分为三个阶段:最初的快速层间交换,然后是反弹,最后是缓慢去除。此外,随着 GR 层间 SO42- 百分比的增加,GR 对 Se(VI)的去除率逐渐降低。研究发现,nFe0@GR对Se(VI)的去除效率是GR的3.53倍。该研究进一步发现,nFe0@GR 与 Se(VI) 反应活性的增强可能是由于在原位形成的 GR 推动了 SO42- 的再平衡。由于与原始 GR 相比,nFe0@GR 与层间 SeO42- 的静电斥力较弱,因此,Se(VI) 可在不反弹的情况下被 nFe0@GR 快速去除。此外,nFe0@GR 还能有效固定模拟地下水中的硒(VI),在降低硒(VI)重新释放到环境中的风险方面具有巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
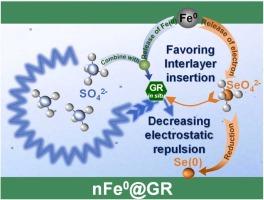
Regulating the interlayer SO42--induced rebound of SeO42- in green rust coupled with iron nanoparticles for groundwater remediation
Green rust (GR) is an interlayer anion-containing Fe(II)/Fe(III) mineral material that is versatile in removing a series of ionic contaminants in water. Taking SeO42- (Se(VI)) as the target contaminant, this study identified that the removal processes of Se(VI) by GR could be divided into three stages: initial rapid interlayer exchange, followed by a rebound, and finally slow removal. In addition, as the percentage of SO42- in GR interlayer increased, the Se(VI) removal by GR gradually decreased. To mediate the SO42--induced rebound of Se(VI), the coupling of GR with iron nanoparticles (nFe0@GR) was proposed in this study and it was found that the removal efficiency of Se(VI) by nFe0@GR was 3.53 folds greater than that of GR. This study further revealed that the enhanced reactivity of nFe0@GR with Se(VI) could be attributed to the re-equilibration of SO42- driven by the formed GR in situ. Since it had a weaker electrostatic repulsion with interlayer SeO42- than pristine GR, the Se(VI) could be quickly removed by nFe0@GR without the rebound. Moreover, the nFe0@GR was demonstrated to be effective in immobilizing Se(VI) from simulated groundwater and has a great potential to reduce the risk of Se(VI) re-release into the environment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


