通过螺旋多芳香族胶囊封装实现远程光学手性转移
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
迄今为止,人们从螺旋状生物结构(如 DNA 和蛋白质)中汲取灵感,广泛创造出螺旋状分子组装体。然而,这种合成螺旋的宿主效用在很大程度上仍未得到充分开发,尤其是在作为光电纳米工具方面。在此,我们报告了新型多芳香族胶囊的制备过程,这种胶囊具有右手或左手四重螺旋,由连接在外表面的手性糖基侧链调节。这种胶囊可在空腔中定量封装非手性荧光染料。通过外层生物手性基团经由四重螺旋壳向内层非手性染料的高效光学手性转移,由此产生的主-客复合物显示出源自结合染料的优异圆偏振发光特性(高达 |glum| = 1.6 × 10-2),这是一种前所未有的手性策略。这种纳米工具可用于球形富勒烯,在溶液中(高达 |gabs| = 1.0 × 10-2)和固态下高效诱导其手性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
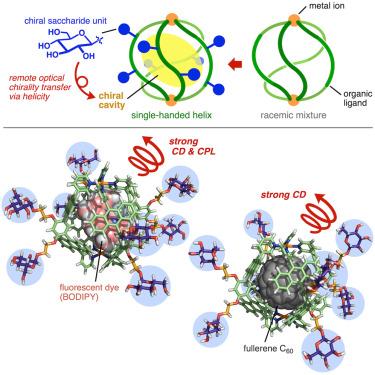

Remote optical chirality transfer via helical polyaromatic capsules upon encapsulation
Helical molecular assemblies have been widely created so far, taking inspiration from helical bioconstructs (e.g., DNAs and proteins). However, the host utilities of such synthetic helices remain largely underdeveloped, particularly as chiroptical nanotools. Here, we report the preparation of new polyaromatic capsules with right- or left-handed quadruple helicity, regulated by chiral saccharide-based side chains attached at the outer surface. The capsule quantitatively encapsulates achiral fluorescent dyes in the cavity. The resultant host-guest complexes display excellent circularly polarized luminescence properties (up to |glum| = 1.6 × 10−2) derived from the bound dyes, through efficient optical chirality transfer from the outer biochiral groups to the inner achiral dyes via the quadruple helical shell, which represents an unprecedented chiroptical strategy. This nanotool can be applied to spherical fullerene to induce its chirality with high efficiency in solution (up to |gabs| = 1.0 × 10−2) and in the solid state.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


