双壳封闭策略提高铂铁钛金属间催化剂在氧还原反应中的耐久性
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
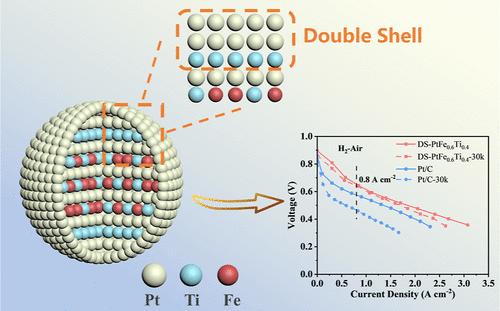
开发在氧还原反应(ORR)中具有更高活性和稳定性的铂基催化剂对于燃料电池的应用至关重要。燃料电池中长期暴露在酸性环境中的 Pt-M(M = 铁、钴、镍、铜等)催化剂会受到过渡金属浸出的影响,导致催化剂加速降解。在此,我们提出了一种双壳封闭策略,通过在铂表皮下引入富钛层来稳定 ORR 催化剂。这种设计旨在防止铁原子的沥滤,从而保护内部的铂铁钛金属间结构。钛的耐酸性和耐腐蚀性使其能够充当物理保护层,抑制铁的沥滤并稳定内部铂铁钛金属间的有序结构。密度泛函理论计算证明,钛层能有效提高铁的空位形成能,从而增强结构的稳定性。双壳 L10-PtFe0.6Ti0.4/P-C 催化剂的质量活性(MA)高达 1.04 A mgPt-1。即使在经过 30,000 个电位循环的加速耐久性测试后,MA 也仅下降了 13.5%。作为燃料电池阴极催化剂,它的峰值功率密度达到了 1.10 W cm-2,在 0.8 A cm-2 时,经过 30,000 次方波电位循环后,电压降仅为 14 mV。这些性能指标超过了能源部 2025 年的目标,也超过了许多代表性催化剂的稳定性数据。此外,这种双壳封闭策略还适用于铂钴基和铂镍基催化剂,证明了其广泛的适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Double-Shell Confinement Strategy Enhancing Durability of PtFeTi Intermetallic Catalysts for the Oxygen Reduction Reaction
The development of Pt-based catalysts with enhanced activity and stability for the oxygen reduction reaction (ORR) is crucial for fuel cell applications. Pt-M (M = Fe, Co, Ni, Cu, etc.) catalysts exposed to prolonged acidic environments in fuel cells suffer from the leaching of transition metals, leading to accelerated catalyst degradation. Here, we present a double-shell confinement strategy to stabilize ORR catalysts by introducing a Ti-rich layer beneath the Pt skin. This design aims to prevent the leaching of Fe atoms, thus protecting the inner PtFeTi intermetallic structure. The resistance of Ti to acid and corrosion allows it to act as a physical protective layer, inhibiting the leaching of Fe and stabilizing the ordered structure of the internal PtFeTi intermetallic. Density functional theory calculations support that the Ti layer can effectively elevate the vacancy formation energy of Fe, thereby enhancing the structural stability. Mass activity (MA) of the double-shell L10-PtFe0.6Ti0.4/P–C catalyst is up to 1.04 A mgPt–1. Even after 30,000 potential cycles of accelerated durability test, the MA decreases by only 13.5%. As the fuel cell cathode catalyst, it achieves a peak power density of 1.10 W cm–2, and the voltage drop at 0.8 A cm–2 is only 14 mV after 30,000 square-wave potential cycles. These performance metrics surpass the DOE 2025 target and exceed the stability data of many of the representative catalysts. Moreover, this double-shell confinement strategy is also applicable to PtCo-based and PtNi-based catalysts, demonstrating its broad applicability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


