动态应变和β-catenin介导的静止间充质基质/干细胞干扰素反应基因抑制作用
IF 2.3
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
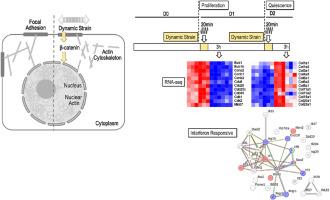
多能骨髓间充质基质/干细胞(间充质干细胞)对机械力做出反应。间充质干细胞通过病灶粘附以及细胞骨架和核内肌动蛋白感知静态和动态力。动态应变会刺激核β-catenin(Ctnnb1),从而控制基因表达并抑制成骨。当间叶干细胞开始表达细胞外基质(ECM)蛋白并产生细胞/细胞接触时,间叶干细胞对外部机械力的敏感性可能会因增殖停止而改变。因此,我们评估了增殖与静止间充质干细胞的基因表达是否以及如何对机械刺激做出反应。我们使用 RNA-seq 和 RT-qPCR 评估了天真(未诱导)间充质干细胞在每天一次的动态应变(200 个周期 × 2 %,20 分钟)后 3 小时的转录组。未经处理的间充质干细胞转录组显示,细胞在第 2 天进入静止期,此时增殖标记下调,而 ECM 相关基因上调。在第 1 天和第 2 天,动态应变都会刺激氧化应激相关基因(如 Nqo1、Prl2c2、Prl2c3)的表达。引人注目的是,在静止间充质干细胞中,我们观察到动态应变抑制了多个干扰素(IFN)反应基因(如 Irf7、Oasl2 和 Isg15)的表达。在使用 siRNA 去除了 β-catenin 的间充质干细胞中,IFN 响应基因被激活,这表明 β-catenin 通常会抑制这些基因。我们的数据表明,动态应变和β-catenin对间叶干细胞中IFN应答基因的功能影响是机理耦合的。由于动态应变和β-catenin降低了间充质干细胞的成骨潜能,我们的研究结果表明,IFN应答基因是新的生物标志物,也是增殖后间充质干细胞在品系承诺早期阶段机械应答的潜在调节因子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dynamic strain and β-catenin mediated suppression of interferon responsive genes in quiescent mesenchymal stromal/stem cells
Multipotent bone marrow mesenchymal stromal/stem cells (MSCs) respond to mechanical forces. MSCs perceive static and dynamic forces through focal adhesions, as well as cytoskeletal and intranuclear actin. Dynamic strain stimulates nuclear β-catenin (Ctnnb1) that controls gene expression and suppresses osteogenesis. The sensitivity of MSCs to external mechanical forces may be altered by cessation of proliferation, when MSCs begin to express extracellular matrix (ECM) proteins and generate cell/cell contact. Therefore, we assessed whether and how gene expression of proliferating versus quiescent MSCs responds to mechanical stimuli. We used RNA-seq and RT-qPCR to evaluate transcriptomes at 3 h after dynamic strain (200 cycles × 2 % for 20 min) once daily during a two-day time course in naïve (uninduced) MSCs. Transcriptomes of untreated MSCs show that cells become quiescent at day 2 when proliferation markers are downregulated, and ECM related genes are upregulated. On both day 1 and day 2, dynamic strain stimulates expression of oxidative stress related genes (e.g., Nqo1, Prl2c2, Prl2c3). Strikingly, in quiescent MSCs, we observe that dynamic strain suppresses multiple interferon (IFN) responsive genes (e.g., Irf7, Oasl2 and Isg15). IFN responsive genes are activated in MSCs depleted of β-catenin using siRNAs, indicating that β-catenin normally suppresses these genes. Our data indicate that the functional effects of dynamic strain and β-catenin on IFN responsive genes in MSCs are mechanistically coupled. Because dynamic strain and β-catenin reduce the osteogenic potential of MSCs, our findings suggest that IFN responsive genes are novel biomarkers and potential regulators of mechanical responses at early stages of lineage-commitment in post-proliferative MSCs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemistry and Biophysics Reports
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
4.60
自引率
0.00%
发文量
191
审稿时长
59 days
期刊介绍:
Open access, online only, peer-reviewed international journal in the Life Sciences, established in 2014 Biochemistry and Biophysics Reports (BB Reports) publishes original research in all aspects of Biochemistry, Biophysics and related areas like Molecular and Cell Biology. BB Reports welcomes solid though more preliminary, descriptive and small scale results if they have the potential to stimulate and/or contribute to future research, leading to new insights or hypothesis. Primary criteria for acceptance is that the work is original, scientifically and technically sound and provides valuable knowledge to life sciences research. We strongly believe all results deserve to be published and documented for the advancement of science. BB Reports specifically appreciates receiving reports on: Negative results, Replication studies, Reanalysis of previous datasets.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


