作为抗阿尔茨海默氏症药物的他克林修饰席夫碱的绿色合成:通过室内和体外分析验证的有效策略
IF 3.8
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
通过无溶剂(绿色)方法开发了多种他克林修饰的希夫碱类似物,并利用 1HNMR、傅立叶变换红外光谱和紫外可见光谱分析对其结构进行了阐明。合成的分子在室温下无需溶剂即可高效生产,产品收率高。随后评估了所开发分子抑制乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BChE)的潜力。这些分子能有效抑制乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BChE),其 IC50 值分别为 0.1 ± 0.02 至 0.3 ± 0.03 μM 和 0.065 ± 0.01 至 0.3 ± 0.03 μM。相比之下,标准药物他克林对 AChE 的 IC50 值为 0.35 ± 0.02 μM,对 BChE 的 IC50 值为 0.1 ± 0.01 μM。值得注意的是,化合物 3f 对这两种酶都有很强的抑制作用。通过分子对接研究,验证并确立了所合成衍生物的结构-活性关系。理论 ADME 研究也预测所有合成的分子都具有极佳的药物相似性。由于氧化应激水平升高与阿尔茨海默病(AD)的认知能力下降有关,因此还对其抗氧化活性进行了评估。这些研究结果表明,该先导化合物可能是治疗阿尔茨海默病的有效抑制剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
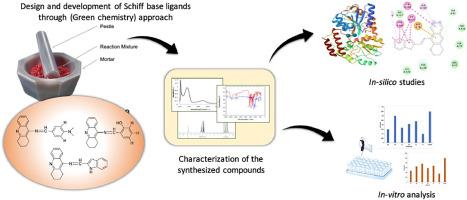
Green synthesis of Tacrine modified Schiff bases as anti-Alzheimer Agents: An effective strategy validated through in-silico and in-vitro analysis
A variety of Tacrine-modified Schiff base analogues were developed via solvent free (green) method and structurally elucidated using 1H![]() NMR, FTIR and UV–Vis analysis. High product yield was obtained from the synthesised molecules, which were produced efficiently at room temperature without the need of a solvent. The developed molecules were subsequently assessed for their potential to inhibit acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). These molecules revealed effective inhibition of AChE and BChE enzymes with IC50 values varying from 0.1 ± 0.02 to 0.3 ± 0.03 μM and 0.065 ± 0.01 to 0.3 ± 0.03 μM respectively. Compared to the standard Tacrine which has IC50 values of 0.35 ± 0.02 μM for AChE and 0.1 ± 0.01 μM for BChE. Notably, compound 3f showed strong inhibition among others for both the enzymes. The structure–activity relationship of derivatives synthesized were verified and established through molecular docking studies. Theoretical ADME studies also predicted excellent drug-likeness for all the synthesized molecules. Antioxidant activities were also assessed because elevated oxidative stress levels are linked with cognitive loss in Alzheimer's disease (AD). These findings suggest that the lead compound is potentially an effective inhibitor for the therapeutic management of AD.
NMR, FTIR and UV–Vis analysis. High product yield was obtained from the synthesised molecules, which were produced efficiently at room temperature without the need of a solvent. The developed molecules were subsequently assessed for their potential to inhibit acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). These molecules revealed effective inhibition of AChE and BChE enzymes with IC50 values varying from 0.1 ± 0.02 to 0.3 ± 0.03 μM and 0.065 ± 0.01 to 0.3 ± 0.03 μM respectively. Compared to the standard Tacrine which has IC50 values of 0.35 ± 0.02 μM for AChE and 0.1 ± 0.01 μM for BChE. Notably, compound 3f showed strong inhibition among others for both the enzymes. The structure–activity relationship of derivatives synthesized were verified and established through molecular docking studies. Theoretical ADME studies also predicted excellent drug-likeness for all the synthesized molecules. Antioxidant activities were also assessed because elevated oxidative stress levels are linked with cognitive loss in Alzheimer's disease (AD). These findings suggest that the lead compound is potentially an effective inhibitor for the therapeutic management of AD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


