揭示离子吸附和介电排斥对通过 pH 值调节的圆柱形纳米孔进行纳米过滤的影响
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
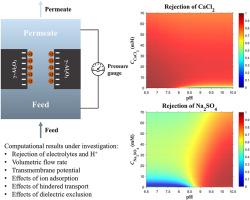
我们通过同时求解修正的纳维-斯托克斯方程、泊松方程和内斯特-普朗克方程,从理论上研究了离子吸附对纳米过滤的影响。考虑到纳米孔中的密闭空间和纳米孔表面附近水的层状结构,分别纳入了受阻传输和各向异性介电排斥,以证明对盐排斥的相应影响。对于对称电解质,离子吸附会略微降低对 NaCl 的排斥,但会显著改变对 CaSO4 的排斥性能。特别是,由于电荷极性反转,离子吸附提高了非对称电解质的析盐性能。在离子吸附作用下,析出物对 pH 值变化的敏感性降低,析出物对进料浓度的依赖性降低。由于进料浓度升高时表面电荷密度增加和离子筛选作用更加明显,因此在特定的 pH 值范围内,CaCl2 和 Na2SO4 的析出量达到最大值。二维表面图说明了抑制作用与浓度和 pH 值的关系。此外,受阻传输的影响也有利于排斥。当离子物种的扩散率下降或共离子(相对于表面电荷极性)与反离子的扩散率之比降低时,排斥作用就会明显增加。最后,纳米孔中的介电排斥作用会导致质子被排斥出表面,从而使表面电荷密度更负,导致等电点向较低的 pH 值移动。垂直介电常数的降低可能会因平行介电常数的增加而增强或部分抵消。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unraveling the impact of ion adsorption and dielectric exclusion on nanofiltration through pH-regulated cylindrical nanopores
We theoretically investigate the effects of ion adsorption on nanofiltration by simultaneously solving the modified Navier-Stokes, Poisson, and Nernst-Planck equations. Considering the confined space in the nanopore and the layered structure of water near the nanopore surface, hindered transport and anisotropic dielectric exclusion are separately incorporated to demonstrate the corresponding influences on salt rejection. For symmetric electrolytes, ion adsorption decreases the rejection of NaCl slightly but alters the rejection performance of CaSO4 significantly. In particular, ion adsorption improves the rejection of asymmetric electrolytes due to the charge polarity reversal. With ion adsorption, the susceptibility of rejection to pH changes diminishes, and the dependence of rejection on feed concentration decreases. Owing to the counteracting effects of increased surface charge density and more pronounced ion screening as feed concentration rises, the rejection of CaCl2 and Na2SO4 exhibits a maximum under specific pH ranges. 2D surface plots illustrate the dependence of rejection on concentration and pH. In addition, the effect of hindered transport is beneficial to rejection. As the diffusivities of ionic species drop or the diffusivity ratio of co-ions (relative to the surface charge polarity) to counterions decreases, the rejection increases pronouncedly. Lastly, dielectric exclusion in the nanopore results in a more negative surface charge density due to the repulsion of protons from the surface, thereby causing a shift of the isoelectric point towards lower pH values. The effect of the reduction in the perpendicular dielectric permittivity may be enhanced or offset partially by that of the increase in the parallel dielectric permittivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Membrane Science
工程技术-高分子科学
CiteScore
17.10
自引率
17.90%
发文量
1031
审稿时长
2.5 months
期刊介绍:
The Journal of Membrane Science is a publication that focuses on membrane systems and is aimed at academic and industrial chemists, chemical engineers, materials scientists, and membranologists. It publishes original research and reviews on various aspects of membrane transport, membrane formation/structure, fouling, module/process design, and processes/applications. The journal primarily focuses on the structure, function, and performance of non-biological membranes but also includes papers that relate to biological membranes. The Journal of Membrane Science publishes Full Text Papers, State-of-the-Art Reviews, Letters to the Editor, and Perspectives.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


