水热法合成用于去除水样中汞离子的绿色 SnS-Carbon 微板吸附剂
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
摘要
汞离子(Hg2+)具有广泛的工业用途,由于它对人类和水生生物都有很大的毒性,因此开发高效的吸附剂来去除汞具有相当重要的意义。本研究介绍了一种由硫化锡(SnS)-碳微板组成的绿色吸附剂,该吸附剂是通过一步水热法直接合成的,随后将其应用于去除水样中的 Hg2+。利用傅立叶变换红外分光光度法 (FT-IR)、场发射扫描电子显微镜 (FESEM)、能量色散 X 射线光谱法 (EDX)、热重分析法 (TGA)、Brunauer-Emmett-Teller (BET) 和 X 射线衍射分析法 (XRD) 对合成的吸附剂进行了表征。通过优化关键参数,并借助 SnS-C 吸附剂的 S 原子(软基)与 Hg2+(软酸)之间的有效相互作用,在 100 mg L-1 Hg2+ 的条件下,Hg2+ 的去除率达到了惊人的 99.0%。对等温线模型的解释表明,Hg2+ 的吸附符合 Langmuir 等温线,最大吸附容量为 238.1 mg/g。最后,SnS-Carbon 微板吸附剂表现出显著的优点,包括绿色便捷的合成路线(通过一步水热法实现)和高效率,使其成为净化水样中 Hg2+ 的有效吸附剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
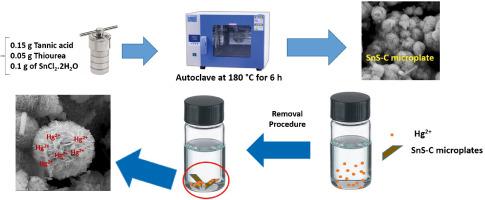
Hydrothermally synthesis of a green SnS-Carbon microplate adsorbent for the removal of mercury ion from water samples
Mercury ion (Hg2+) finds a broad industrial application and due to its significant toxicity to both humans and aquatic life, development of highly effective adsorbents for its removal holds considerable importance. This study introduces a green adsorbent consisting of tin sulfide (SnS)‑carbon microplates synthesized through a straightforward one-step hydrothermal process followed by its application for the removal of Hg2+ from water samples. The synthesized adsorbent is characterized using Fourier Transform Infrared Spectrophotometry (FT-IR), Field Emission Scanning Electron Microscopy (FESEM), Energy-Dispersive X-ray spectroscopy (EDX), Thermogravimetric analysis (TGA), Brunauer–Emmett–Teller (BET) and X-Ray diffraction analysis (XRD). Through the optimization of crucial parameters and with thanks to the effective interaction between S atoms (soft base) of SnS-C adsorbent and Hg2+ (soft acid), an impressive Hg2+ removal percentage of approximately 99.0 % is achieved for 100 mg L−1 Hg2+. Interpreting of isotherm models indicate that Hg2+ adsorption conforms to the Langmuir isotherm with a maximum adsorption capacity of 238.1 mg/g. Finally, the SnS-Carbon microplate adsorbent exhibits notable advantages, including a green and convenient synthesis route (achieved through a one-step hydrothermal method) and high efficiency, making it a potent adsorbent for the decontamination of Hg2+ from water samples.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


