分子探针的光化学和电化学行为:检测具有不同反应速率的多种离子(Cu2+、ClO- 和 CN-)的荧光开关响应
IF 4.1
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
一种含有苯基取代咪唑单元和二氨基马来腈(DAMN)分子的分子探针 3 已被合成并表征出来。在部分水介质(ACN/H2O,9.5:0.5 v/v)中对探针 3 的光物理和电化学行为进行了研究。由于分子内电荷转移(ICT)过程的变化,探针 3 与不同种类的离子相互作用时,对 Cu2+、ClO- 和 CN- 离子表现出不同的灵敏度,反应时间和反应速率也不同。Cu2+ 和 ClO- 离子在介质中因水解亚胺键形成化合物 2 而显示出更强的发射,而 CN- 离子则因 -OH/-NH2 功能的去质子化而观察到荧光淬灭。探针的动力学和光学行为表明,与 Cu2+ 离子(络合和水解;absk1,2,3(Cu2+) = 1.17、0.74 和 0.06 s-1)和 ClO- 离子(水解和去质子化;absk1,2(ClO-) = 3.0 和 0.60 s-1)的相互作用分两步进行,而与 CN- 离子(absk1,2(CN-) = 2.13 和 1.66 s-1)的去质子化作用只需一步。探针 3 与 ClO- 的相互作用比与 Cu2+/CN- 离子的相互作用快。探针 3 与离子之间的约伯图分析表明,两者的化学计量比分别为 1:1(Cu2+/ClO-)和 1:2(CN-),具有良好的检测限(nM)和结合亲和力(LOD,17;KCu2+ = 3.23 × 106 M-1;LOD,18.5;KClO- = 5.23 × 105 M-1 和 LOD,23.2;KCN- = 5.50 × 109 M-2)。探针 3 显示出肉眼灵敏的荧光反应,可检测试纸条上的受测离子,并在实际水样分析中显示出良好的离子回收率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
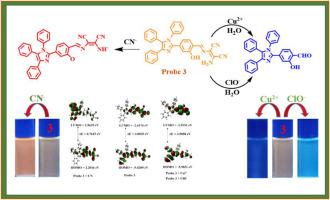
Photochemical and electrochemical behavior of a molecular probe: Fluorescence on-off-on response to detect multiple ions (Cu2+, ClO− and CN−) with different rate of reaction
A molecular probe 3 containing phenyl substituted imidazolyl unit and diaminomaleonitrile (DAMN) moiety has been synthesized and characterized. Photophysical and electrochemical behavior probe 3 were studied in partial aqueous medium (ACN/H2O, 9.5:0.5 v/v). Probe 3 upon interaction with different class of ions showed sensitivity for Cu2+, ClO− and CN− ions with different response time and reaction rates due to variation in intramolecular charge transfer (ICT) process. Both Cu2+ and ClO− ions displayed enhanced emission due to hydrolysis of imine bond to form compound 2 in the medium whereas, with CN− fluorescence quenching was observed due to the deprotonation of –OH/–NH2 functions. The kinetic and optical behavior of the probe suggested that interaction occurred in two steps with Cu2+ (complexation and hydrolysis; absk1,2,3(Cu2+) = 1.17, 0.74 and 0.06 s−1) and ClO− (hydrolysis and deprotonation; absk1,2(ClO−) = 3.0 and 0.60 s−1) ions while CN− (absk1,2(CN−) = 2.13 and 1.66 s−1) induces deprotonation in one step. The interaction of probe 3 with ClO− was relatively fast than with Cu2+/CN− ions. Job's plot analysis between probe 3 and ions revealed a 1:1 (Cu2+/ClO−) and 1:2 (CN−) ratio stoichiometry with good limit of detection (nM) and binding affinity (LOD, 17; KCu2+ = 3.23 × 106 M−1; LOD, 18.5; KClO− = 5.23 × 105 M−1 and LOD, 23.2; KCN− = 5.50 × 109 M−2) respectively. Probe 3 displayed a naked–eye sensitive fluorescence response to detect tested ions on test paper strips and good recovery percentage of ions in real water sample analysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


