生物螯合剂对咸水养殖水中镉和铅的同时封存:机理分析
IF 5.4
Q2 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
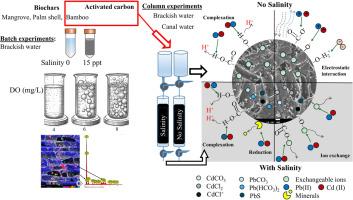
沿海水产养殖面临着金属污染的挑战,尤其是铅(Pb)和镉(Cd)的污染。本研究在批量实验中考察了盐度和溶解氧(DO)对铅和镉与竹子(BB)、红树林(MB)和棕榈壳(PSB)生物炭相互作用的协同效应。在单一(铅或镉)和双溶质(铅+镉)实验室规模的咸水系统中,比较了生物炭与活性炭(AC)的性能。吸附研究表明,生物炭和 AC 对铅和镉的吸附遵循 Freundlich 吸附模型,但 BB 除外,在 15 ppt 盐度条件下遵循 Langmuir 模型。溶解氧的增加通过影响生物炭表面电荷而略微促进了铅和镉的吸附,而盐度的增加则对吸附产生了负面影响。在各种生物炭中,BB 表现出最高的铅和镉吸附能力。选择 BB 和 AC 对合成咸水和运河水进行柱实验。吸附数据符合克拉克模型,强调了离子交换和多层吸附模式的作用。盐度的增加降低了可交换部分,同时增加了碳酸盐结合部分、可还原部分和可氧化部分,这表明离子交换和氧化物之间存在相互作用。SEM-EDS 和 XRF 分析证实,BB 和 AC 上都存在吸附的铅和镉。在去除运河水中的铅和镉方面,BB 比 AC 更有效,没有观察到解吸现象。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Simultaneous sequestration of cadmium and lead in brackish aquaculture water by biochars: A mechanistic insight
Coastal aquaculture faces metal pollution challenges, particularly from lead (Pb) and cadmium (Cd). This study examined the synergistic effects of salinity and dissolved oxygen (DO) on Pb and Cd interactions with biochars from bamboo (BB), mangrove (MB) and palm shell (PSB) in batch experiments. The performance of biochars was compared to activated carbon (AC) in single (Pb or Cd) and bi-solute (Pb + Cd) lab-scale brackish water systems. Adsorption studies showed that Pb and Cd adsorptions onto biochars and AC followed the Freundlich adsorption model, except for BB, which followed the Langmuir model at 15 ppt salinity. The increase in DO slightly facilitated the adsorption of Pb and Cd by influencing biochar surface charge, whereas increases in salinity negatively affected adsorption. Among the biochars, BB exhibited the highest Pb and Cd adsorption capacity. BB and AC were selected for column experiments with synthetic brackish water and canal water. The adsorption data fitted the Clark model, emphasizing the role of ion exchange and the multilayer pattern of adsorption. Increased salinity decreased the exchangeable fraction while increasing carbonate-bound, reducible, and oxidizable fractions, suggesting ion exchange and oxide interactions. SEM-EDS and XRF analyses confirmed the presence of adsorbed Pb and Cd on both BB and AC. BB demonstrated to be more effective than AC in removing Pb and Cd from canal water with no desorption observed and it can be a cost-effective alternative to sequester Pb and Cd from shrimp nursery ponds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of hazardous materials advances
Environmental Engineering
CiteScore
4.80
自引率
0.00%
发文量
0
审稿时长
50 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


