以Fe(NO3)3-9H2O为促进剂、亚硝基和硝基源的(异)烷的区域选择性C(sp2)-H亚硝化和硝化反应
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
利用经济、无毒的 Fe(NO3)3.9H2O 作为亚硝基/硝基源,成功开发了一种具有区域选择性的 C(sp2)-H 亚硝基化和硝化方法,用于修饰各种非定向生物活性分子。该方法在无酸和易于操作的条件下进行,具有良好的区域选择性和广泛的底物范围(38 个实例;收率高达 90%)。该反应可在克级规模上进行,且效率很高,在药物和材料分子的合成和改性方面具有很高的实用性。详细的机理研究表明,亚硝基或硝基产物的形成可能是通过芳基自由基阳离子中间体与亚硝基或硝基自由基的自由基交叉偶联进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
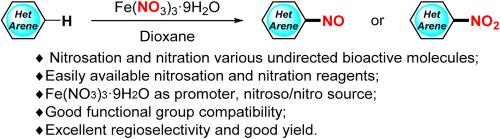
Regioselective C(sp2)-H nitrosation and nitration of (Hetero)arenes with Fe(NO3)3·9H2O as promoter, nitroso and nitro source
A regioselective C(sp2)-H nitrosation and nitration protocol has been successfully developed for the modification of various undirected bioactive molecules by using the economical and nontoxic Fe(NO3)3.9H2O as nitroso/nitro source. This method is conducted under acid-free and ease of handling conditions and is featured with good regioselectivity as well as a broad substrate scope (38 examples; yields up to 90 %). This reaction could be conducted on a gram scale with good efficiency, demonstrating high utility for the synthesis and modification of drug and material molecules. Detailed mechanistic studies indicate that the formation of the nitroso or nitro products might be proceeded via the radical cross coupling of aryl radical cation intermediates with nitroso or nitro radical.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


