烷基 AMP 酯的改进合成法
IF 16.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
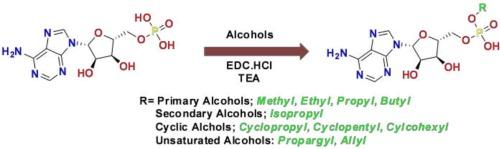
属于乙酰-CoA/NRPS/荧光素酶(ANL)超家族的酶是药物开发的重要目标。乙酰-CoA 合成酶(ACS)就是这个超家族的成员之一。这种酶是治疗各种传染性疾病和癌症的新兴靶点。烷基 AMP 酯已成为 ACS 的强效抑制剂。以前合成这些酯类的方法通常涉及水基反应,或者必须通过反相预高效液相色谱法或离子交换色谱法进行纯化。为了解决这些难题,我们开发了一种新方法,利用 1-乙基-3-(3-二甲基氨基丙基)碳二亚胺盐酸盐(EDC-HCl)将 5′-腺嘌呤核苷酸与相应的醇偶联,并以三乙胺为碱。这种方法能以极高的产率获得伯醇、仲醇、不饱和醇和环醇。重要的是,我们优化了反应条件,无需反相预液相色谱法或离子交换色谱法就能获得极高的产率和纯度。取而代之的是使用 Biotage ® Sfar 球形硅胶进行硅胶色谱纯化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
An improved synthesis of alkyl AMP esters
Enzymes belonging to the Acyl-CoA/NRPS/Luciferase (ANL) superfamily of enzymes, are significant targets in drug development. One member of this superfamily is Acetyl-CoA Synthetase (ACS). This enzyme is an emerging target for the treatment of various infectious diseases and cancer. Alkyl AMP esters have emerged as potent inhibitors of ACS. Previous methods for synthesizing these esters often involved water-based reactions or necessitate purification through reverse phase prep-HPLC or ion-exchange chromatography. To address these challenges, we developed a new approach utilizing 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl) to couple 5′-adenylic acid to the corresponding alcohol, utilizing triethylamine as the base. This method yielded primary, secondary, unsaturated, and cyclic alcohols in excellent yields. Importantly, we optimized the reaction conditions to achieve excellent yield and purity without the need for reverse phase prep-HPLC or ion exchange chromatography. Instead, purification was achieved through silica gel chromatography using Biotage ® Sfar spherical silica gel.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Accounts of Chemical Research
化学-化学综合
CiteScore
31.40
自引率
1.10%
发文量
312
审稿时长
2 months
期刊介绍:
Accounts of Chemical Research presents short, concise and critical articles offering easy-to-read overviews of basic research and applications in all areas of chemistry and biochemistry. These short reviews focus on research from the author’s own laboratory and are designed to teach the reader about a research project. In addition, Accounts of Chemical Research publishes commentaries that give an informed opinion on a current research problem. Special Issues online are devoted to a single topic of unusual activity and significance.
Accounts of Chemical Research replaces the traditional article abstract with an article "Conspectus." These entries synopsize the research affording the reader a closer look at the content and significance of an article. Through this provision of a more detailed description of the article contents, the Conspectus enhances the article's discoverability by search engines and the exposure for the research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


