铁(III)-金属酸络合物在羊毛上的染色行为与 pH 值相关的铁(III)-络合物化学计量的关系
IF 4.1
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
铁与多酚(如五倍子单宁和黄酮类化合物)的络合物自古以来就被用于纺织品着色、绘画和制备墨水。历史文物上的铁胆墨水成分已通过现代仪器方法进行了研究。然而,铁与多酚的络合物的染色行为仍然基于经验知识。本研究将没食子酸铁(III)络合物作为模型系统,以阐明它们在羊毛上随 pH 值变化的吸附和染色行为。对染浴中存在的络合物种类进行的模型计算表明,在 pH 值为 3 时,主要存在二元络合物 [Fe(III)(GA2-)]+,而在 pH 值为 4 时,主要存在三元络合物 [Fe(III)(GA2-)2]-。染料吸附作用的基础是范德华力,而 Fe(III)- 复合物与角蛋白之间的离子相互作用关系不大。所阐述的理论模型还解释了在铁盐存在的情况下,五倍子单宁、多酚和黄酮类化合物在染色时染浴 pH 值的影响,因此对提高这些天然着色剂的利用率具有特别重要的意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
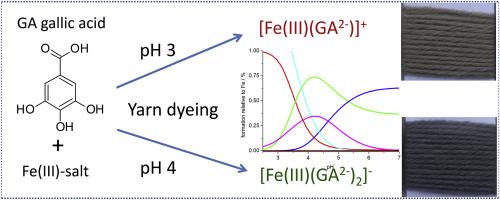
Dyeing behaviour of iron(III)-gallic acid complexes on wool as function of pH-dependent iron(III)-complex stoichiometry
Iron complexes with polyphenolics e.g. gallotannins and flavonoids have been used since ancient times for coloration of textiles, painting and preparation of inks. The composition of iron gall inks on historical artefacts has been investigated with modern instrumental methods. However the dyeing behaviour of iron-complexes with polyphenols still bases on empirical knowledge. In this study Fe(III)-complexes with gallic acid were used as model system to elucidate their pH dependent sorption and dyeing behaviour on wool. At dyebath pH 3 high sorption of Fe(III)-gallic acid complexes was observed, however darker dyeings were obtained at dyebath pH 4. Model calculations for the complex species present in the dyebath indicated the presence of the binary complex [Fe(III)(GA2−)]+ as main species at pH 3, while at pH 4 the ternary species [Fe(III)(GA2−)2]- prevails. The dye sorption bases on Van-der-Waals forces, while ionic interactions between Fe(III)-complex and keratin are of minor relevance. The elaborated theoretical model also explains the influence of dyebath pH in dyeing with gallotannins, polyphenols and flavonoids in presence of iron salts and thus is of particular relevance for improved utilisation of these natural colorants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


