发现四氮唑硫醚:使用二月桂碘鎓盐进行高效、环保和无金属的 S-芳基化反应
IF 5.8
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究开发了一种高效、环保、无金属的方法,利用二月桂碘鎓盐作为芳基转移试剂,通过四氮唑-5-硫醚的 S-芳基化反应合成官能化四氮唑硫醚。这种新方法可在简便的条件下快速获得四氮唑硫醚衍生物。我们的研究强调了不对称二芳基二碘鎓盐的化学选择性,强调了它们对立体受阻和电子缺陷芳基转移的偏好。值得注意的是,合成的化合物 3h 对 A2780(卵巢癌)和 MDA-MB-231(乳腺癌)肿瘤细胞具有抗肿瘤活性。硅学研究预测这些化合物具有良好的药物相似性和较低的毒性风险。这些发现凸显了官能化四氮唑硫醚作为抗增殖剂的潜力,为今后的进一步开发和优化研究铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
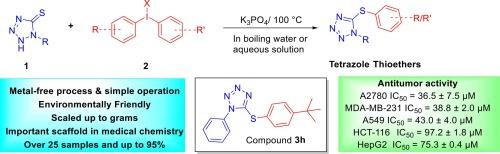
Discovery of tetrazole thioethers: An efficient, environmentally friendly and metal-free S-arylation using diaryliodonium salts
An efficient, environmentally friendly, and metal-free method for the synthesis of functionalized tetrazole thioethers via the S-arylation of tetrazole-5-thiones using diaryliodonium salts as aryl transfer reagents has been developed. This novel methodology provides rapid access to tetrazole thioether derivatives under facile conditions. Our study underscores the chemoselectivity of unsymmetrical diaryliodonium salts, emphasizing their preference for the transfer of sterically hindered and electron-deficient aryl groups. Notably, the synthesized compound 3h exhibits antitumor activity against A2780 (ovarian cancer) and MDA-MB-231 (breast cancer) tumor cells. In silico studies predict these compounds to possess good drug-likeness and low toxicity risk. These findings highlight the potential of functionalized tetrazole thioethers as antiproliferative agents and pave the way for further development and optimization in future investigations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Saudi Chemical Society
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
8.90
自引率
1.80%
发文量
120
审稿时长
38 days
期刊介绍:
Journal of Saudi Chemical Society is an English language, peer-reviewed scholarly publication in the area of chemistry. Journal of Saudi Chemical Society publishes original papers, reviews and short reports on, but not limited to:
•Inorganic chemistry
•Physical chemistry
•Organic chemistry
•Analytical chemistry
Journal of Saudi Chemical Society is the official publication of the Saudi Chemical Society and is published by King Saud University in collaboration with Elsevier and is edited by an international group of eminent researchers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


