低价锰活性位点:深入了解与磺化蒽醌染料的强化相互作用以及铁改性隐色烷的动力学吸附研究
IF 5.5
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
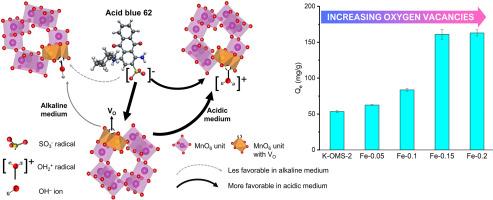
本研究提出了一种面部共沉淀方法,用于富集掺铁隐色烷的低价锰位点。傅立叶变换红外光谱显示,Mn3+-OH 键在 1041 和 1116 cm-1 处的振动比制备的材料明显增强。X 射线衍射、扫描电子显微镜、拉曼光谱、氧的温度编程解吸和电感耦合等离子体质谱分析结果都验证了掺铁隐色烷上氧空位的增加。当初始 pH 值为 5.7 时,吸附容量(Qe,毫克 AB62/吸附剂)从 54 ± 1.3 毫克/克(无掺杂隐色兰)增加到 161 ± 6.7 毫克/克(Fe-0.15)时,实验证明了 Mn3+-OH 位点在吸附去除酸性蓝 62(AB62)中的重要作用。与 Fe-0.15 相比,Qe 从 313 毫克/克(初始 pH 值为 3.70)降至 67 毫克/克(初始 pH 值为 9.95),这表明在酸性介质中发生了质子化,而在碱性介质中发生了去质子化,反映出 Mn3+-OH 位点与磺酸盐基团的相互作用得到了有效加强。在 Fe-0.15 上,吸附后 1041 和 1116 cm-1 处的尖带消失,1250 cm-1 处的宽带补充,这表明磺酸基团被 -OH 物种(来自 Mn3+-OH 位点)取代。此外,吸附后 1187 和 1230 cm-1 处 O=S=O 的两种伸展模式变差,表明形成了单齿或双齿复合物。动力学研究证实,AB62 在 Fe-0.15 上的化学吸附与伪二阶动力学、埃洛维奇和朗缪尔等温线模型兼容。目前的研究结果首次证明了 AB62 在掺铁的隐色兰上的化学吸附作用,以及 Fe-0.15 是去除磺化蒽醌染料的可行吸附剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Low-valent manganese active sites: Insight into reinforced interaction with sulfonated anthraquinone dye and kinetic adsorption studies over iron-modified cryptomelane
This study presents a facial co-precipitation method to enrich low-valent manganese sites for iron-doped cryptomelane. Fourier-transform infrared spectroscopy exhibits a noticeable enhancement of both vibrations at 1041 and 1116 cm-1 ascribed to Mn3+-OH bond over as-prepared materials. X-ray diffraction, scanning electron microscopy, Raman spectroscopy, the temperature-programmed desorption of oxygen and inductively coupled plasma-mass spectrometry results all verify the increase in oxygen vacancies on iron-doped cryptomelane. The vital role of Mn3+-OH sites for adsorptive removal of acid blue 62 (AB62) was experimentally evident when adsorption capacity (Qe, mgAB62/gadsorbent) increased from 54 ± 1.3 mg/g (for non-doped cryptomelane) to 161 ± 6.7 mg/g (for Fe-0.15) at initial pH 5.7. The decrease of Qe from 313 mg/g (for initial pH 3.70) to 67 mg/g (for initial pH 9.95) over Fe-0.15 suggests protonation in acid media and deprotonation in basic media, reflecting efficient Mn3+-OH sites for reinforced interaction with sulfonate groups. The disappearance of sharp bands at 1041 and 1116 cm-1 after adsorption and the replenishment of a broad band at ∼1250 cm-1 over Fe-0.15 demonstrate the displacement of sulfonate groups by -OH species (from Mn3+-OH sites). Moreover, the deterioration of two stretching modes for O=S=O at 1187 and 1230 cm-1 after adsorption reveals the formation of a monodentate or bidentate complex. Kinetic studies confirm the compatibility of AB62 chemisorption over Fe-0.15 with the pseudo-second-order kinetic, Elovich, and Langmuir isotherm models. The current findings first support evidences for the AB62 chemisorption on iron-doped cryptomelane and a Fe-0.15-feasible adsorbent for removal of sulfonated anthraquinone dye.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal Advances
Engineering-Industrial and Manufacturing Engineering
CiteScore
8.30
自引率
0.00%
发文量
213
审稿时长
26 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


