基于神经成像的阿尔茨海默病淀粉样蛋白清除疗法药物研发,采用验证因果关系分析法
IF 2.1
4区 医学
Q3 CLINICAL NEUROLOGY
引用次数: 0
摘要
衰老引起的肝功能障碍会损害胆固醇代谢,降低大脑中胆酸(CA,胆汁酸)的可用性。据报道,在阿尔茨海默病(AD)的临床前研究中,CA 具有神经保护特性。我们的目的是探究淀粉样蛋白、胆酸和脑血流这三者之间的因果联系,从而探索对阿尔茨海默病的治疗可行性。我们从 AD 神经影像计划生物样本平台评估了血清胆酸(182 名健康人/136 名 AD 患者)。我们还评估了 50 名健康受试者和 50 名阿尔茨海默氏症受试者,包括 MRI-ASL 扫描(脑血流,CBF)和 PET-AV45 扫描(淀粉样蛋白负荷)。我们进行了计算因果连接,以确定参数之间的因果关系。AD受试者的血清胆酸大幅下降,仅为对照组的一半。因果连通性揭示了两条新的因果途径:(i) 血清胆酸的减少明显增加了淀粉样蛋白负荷;(ii) 淀粉样蛋白负荷的增加明显降低了CBF。我们通过临床前的旁证观察分别证实了这两条致病途径:(a)增加胆酸可通过减少γ-分泌酶来减少淀粉样蛋白的形成;(b)淀粉样蛋白的减少可通过松弛血管周细胞来诱导毛细血管流量的增加。事实上,胆酸可增加淀粉样蛋白清除因子。基于神经影像学的因果连通性分析表明,胆酸衍生物重新定位的药理调节可能具有巨大的潜力,可作为治疗注意力缺失症的新窗口。现有的治疗试验线索提供了指示性临床验证。本文章由计算机程序翻译,如有差异,请以英文原文为准。
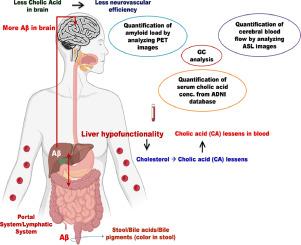
Neuroimaging-based drug discovery for amyloid clearance therapy in Alzheimer's disease using validated causation analysis
Aging-induced hepatic dysfunction can impair cholesterol metabolism, reducing the availability of cholic acid (CA, bile-acid) in brain. CA is reported to have neuroprotective characteristics in preclinical investigations of Alzheimer's disease (AD). Our aim is to probe the causal-connectivity between the players: amyloid, cholic acid and cerebral-blood-flow, and thereby explore therapeutic applicability in AD. From AD neuroimaging initiative biospecimen platform, we evaluated serum cholic-acid (182 healthy/136 AD individuals). We also assessed 50 healthy/50 Alzheimer's subjects containing MRI-ASL scanning (cerebral blood-flow, CBF) and PET-AV45 scanning (amyloid-load). We performed computational causal connectivity to determine the cause-effect relationship among the parameters. Serum cholic acid in AD subjects substantially decreased to half of controls. Causal-connectivity revealed two novel causative pathways: (i) Decreasing serum CA markedly increased amyloid-load; (ii) Increasing amyloid-load distinctly decreased CBF. We substantiated these two causation pathways respectively with collateral available preclinical observations: (a) increased cholic acid reduces amyloid formation by diminishing gamma-secretase; (b) this decreased amyloid induces capillary-flow enhancement by relaxing vascular pericytes. Indeed, cholic acid can increase amyloid-clearance factor. Neuroimaging-based causal connectivity analysis showed that repositioned pharmacological modulation by cholate derivatives may have appreciable potential as novel window for therapeutic approach to AD. Indicative clinical validation is furnished from available therapeutic trial leads.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.80
自引率
0.00%
发文量
86
审稿时长
22.5 weeks
期刊介绍:
The Neuroimaging section of Psychiatry Research publishes manuscripts on positron emission tomography, magnetic resonance imaging, computerized electroencephalographic topography, regional cerebral blood flow, computed tomography, magnetoencephalography, autoradiography, post-mortem regional analyses, and other imaging techniques. Reports concerning results in psychiatric disorders, dementias, and the effects of behaviorial tasks and pharmacological treatments are featured. We also invite manuscripts on the methods of obtaining images and computer processing of the images themselves. Selected case reports are also published.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


