在有限空间内定制 5 纳米以下的掺铁 CeO2 纳米晶体,促进可见光条件下的光催化氢演化
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
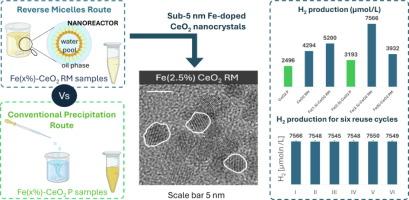
这项工作旨在研究反向胶束(RM)制备路线在合成亚 5 纳米掺铁 CeO2 纳米晶体中的效率,以提高甲醇水溶液在可见光驱动下的光催化制氢能力。通过广泛的物理化学表征,评估了将沉淀反应限制在胶束笼中的有效性。特别是,ICP-MS 分析确定了标称成分(0-5 摩尔% 铁),X 射线衍射证明了不存在单独的含铁结晶相。利用紫外-可见光谱、拉曼光谱和光致发光光谱研究了有效的别价掺杂和对光学特性的调节。研究发现,2.5 摩尔% 的铁含量是显著降低带隙、提高氧空位缺陷浓度和增加电荷载流子寿命的最佳含量。研究了用 RM 制备不同铁含量的铁掺杂 CeO2 的光催化活性,并与未掺杂 CeO2 进行了比较。结果表明,最佳铁含量为 2.5 摩尔%,产氢量最高(在可见光下 240 分钟后为 7566 μmol L-1)。此外,为了进行比较,还采用了传统的沉淀法(P)来制备最佳含铁量(2.5 摩尔% Fe)的含铁 CeO2。RM 法制备的掺铁 CeO2 催化剂的产氢量明显高于 P 法制备的样品。用 RM 法制备的最佳掺铁 CeO2 在六个重复使用周期内都很稳定。此外,通过在 D2O 存在下的测试,研究了水在可见光下光催化氢气进化机理中的作用。结果表明,氢是由导带中的电子促进 H+ 还原产生的,而甲醇则优先被光生正电子空穴氧化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tailoring sub-5 nm Fe-doped CeO2 nanocrystals within confined spaces to boost photocatalytic hydrogen evolution under visible light
This work aimed to study the efficiency of the reverse micelle (RM) preparation route in the syntheses of sub-5 nm Fe-doped CeO2 nanocrystals for boosting the visible-light-driven photocatalytic hydrogen production from methanol aqueous solutions. The effectiveness of confining precipitation reactions within micellar cages was evaluated through extensive physicochemical characterization. In particular, the nominal composition (0–5 mol% Fe) was preserved as ascertained by ICP-MS analysis, and the absence of separate iron-containing crystalline phases was supported by X-ray diffraction. The effective aliovalent doping and modulation of the optical properties were investigated using UV-Vis, Raman, and photoluminescence spectroscopies. 2.5 mol% iron was found to be an optimal content to achieve a significant decrease in the band gap, enhance the concentration of oxygen vacancy defects, and increase the charge carrier lifetime. The photocatalytic activity of Fe-doped CeO2 prepared at different Fe contents with RM preparation was studied and compared with undoped CeO2. The optimal iron load was identified to be 2.5 mol%, achieving the highest hydrogen production (7566 μmol L−1 after 240 min under visible light). Moreover, for comparison, the conventional precipitation (P) method was adopted to prepare iron containing CeO2 at the optimal content (2.5 mol% Fe). The Fe-doped CeO2 catalyst prepared by RM showed a significantly higher hydrogen production than that obtained with the sample prepared by the P method. The optimal Fe-doped CeO2, prepared by the RM method, was stable for six reuse cycles. Moreover, the role of water in the mechanism of photocatalytic hydrogen evolution under visible light was studied through the test in the presence of D2O. The obtained results evidenced that hydrogen was produced from the reduction of H+ by the electrons promoted in the conduction band, while methanol was preferentially oxidized by the photogenerated positive holes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


