用于锂电池有效过热保护的可逆相变聚(甲基丙烯酸苄酯)/离子液体电解质
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
电池安全受到各种因素的影响,其中热失控是最令人担忧的问题之一。虽然大多数研究都集中于开发一次性自激活热保护机制,如温度响应电极和热关断分离器,但这些方法只能提供不可逆的保护。最近,可逆温敏电解质作为一种有前途的替代品出现了,它同时具有热可逆性和自我保护特性。然而,进一步的研究对于全面了解这些热关断电解质至关重要。在本研究中,我们提出了较低临界溶液温度(LCST)相行为聚(甲基丙烯酸苄酯)/咪唑基离子液体混合物,用于制备温度敏感型电解质,为电池提供可逆的热关断保护。这种电解质具有适当的保护温度(∼105 °C),并能在 105 °C下的 1 分钟内快速反应,使电池在电压突然降至 3.38 V 时几乎不放电,并在 30 分钟内提供有效的热关断保护。冷却至室温后,电池即可恢复原有性能。此外,这种电解液还具有出色的循环稳定性,在循环 500 次后,电池容量保持率达到 91.6%。这项研究为防止电池因过热而引发热失控提供了可行的解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
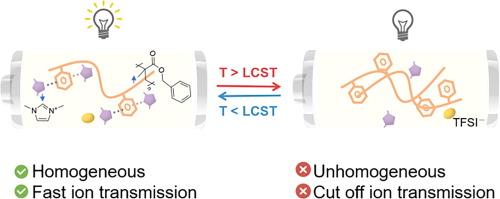
Reversible phase transition poly(benzyl methacrylate)/ionic liquid electrolytes for effective overheating protection in lithium batteries
Battery safety is influenced by various factors, with thermal runaway being one of the most significant concerns. While most studies have concentrated on developing one-time, self-activating mechanism for thermal protection, such as temperature-responsive electrodes, and thermal-shutdown separators, these methods only provide irreversible protection. Recently, reversible temperature-sensitive electrolytes have emerged as promising alternatives, offering both thermo-reversibility and self-protective properties. However, further research is crucial to fully understand these thermal-shutdown electrolytes. In this study, we propose lower critical solution temperature (LCST) phase behavior poly(benzyl methacrylate)/imidazolium-based ionic liquid mixtures to prepare temperature-sensitive electrolytes that provide reversible thermal shutdown protection of batteries. This electrolyte features an appropriate protection temperature (∼105 °C) and responds quickly within a 1 min at 105 °C, causing cells to hardly discharge as the voltage suddenly drops to 3.38 V, and providing efficient thermal shutdown protection within 30 min. Upon cooling back to room temperature, the battery regains its original performance. Additionally, the electrolyte exhibits excellent cycling stability with the capacity retention of the battery is 91.6% after 500 cycles. This work provides a viable solution for preventing batteries from thermal runaway triggered by overheating.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


