功能性三元盐结构可为超稳定全固态锂金属电池提供原位 Li3N/LiF 富集界面
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
基于聚(环氧乙烷)的聚合物全固态锂金属电池(ASSLBs)因其低成本、良好的加工能力和高能量密度而受到广泛关注。然而,锂枝晶在聚合物固态电解质中的生长破坏了锂阳极的可逆性,仍然阻碍着其广泛应用。一种有效的策略是构建一个优异的固态电解质界面。在这里,锂阳极和三元盐电解质之间原位形成了富含 Li3N 和 LiF 的稳定界面。这种三元盐电解质是通过在聚环氧乙烷基体中引入双(三氟甲烷磺酰)亚胺锂(LiTFSI)、双(氟磺酰)亚胺锂(LiFSI)和 LiNO3 而创新设计的。表面表征结果表明,LiNO3 和 LiFSI 有助于形成 Li3N-LiF 富集界面,同时 LiTFSI 确保了优异的导电性。从理论上讲,在各种锂化合物成分中,Li3N 具有较高的离子电导率,有利于降低过电位,而 LiF 具有较高的界面能,可以提高成核能,抑制锂枝晶的形成。实验结果表明,与磷酸铁锂阴极耦合的 ASSLBs 在 2 C 温度下循环约 2200 次后,显示出极其出色的循环稳定性,最终相应的放电比容量为 96.7 mA h g-1。此外,还提出了一个关于 Li3N-LiF 接口工作机制的示意图。本文章由计算机程序翻译,如有差异,请以英文原文为准。
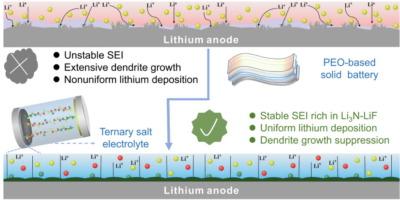
Functional ternary salt construction enabling an in-situ Li3N/LiF-enriched interface for ultra-stable all-solid-state lithium metal batteries
Poly(ethylene oxide)-based polymer all-solid-state lithium metal batteries (ASSLBs) have received widespread attention due to their low cost, good process ability, and high energy density. Nevertheless, the growth of Li dendrites within polymer solid-state electrolytes damages the reversibility of Li anodes and still impedes their widespread application. One efficient strategy is to construct a superior solid electrolyte interface. Herein, a stable interface enriched with Li3N and LiF is in-situ formed between Li anode and a ternary salt electrolyte. This ternary salt electrolyte is innovatively designed by introducing lithium bis(trifluoromethane sulfonyl)imide (LiTFSI), lithium bis(fluorosulfonyl)imide (LiFSI), and LiNO3 to poly(ethylene oxide) matrix. Surface characterization indicates that LiNO3 and LiFSI contribute to forming a Li3N-LiF-enriched interface and meanwhile LiTFSI ensures excellent conductivity. Theoretically, among various Li compound components, Li3N has high ionic conductivity, which is beneficial for reducing overpotential, while LiF has high interfacial energy which can enhance nucleation energy and suppress the formation of Li dendrites. The experimental results show that ASSLBs coupled with LiFePO4 cathode display extremely excellent cycle stability approximately 2200 cycles at 2 C, with a final and corresponding discharge specific capacity of 96.7 mA h g−1. Additionally, a schematic illustration of the working mechanism for the Li3N-LiF interface is proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


