通过一步湿化学反应实现稳定富锂层状氧化物界面的双重策略,实现长氧氧化还原可逆性
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
高电压下的氧释放和电解质分解会无休止地加剧高能量密度富锂层状氧化物(LLO)的界面恶化和结构退化,从而导致电压和容量衰减。在这里,通过室温下的一步湿化学反应,在 LLO 表面同时实现了 CrxB 复合物涂层和局部梯度掺杂的双重策略。密度泛函理论(DFT)计算证明,通过局部梯度掺杂形成稳定的B-O和Cr-O键,可显著降低界面晶格O的高能O 2p态,这对近表面晶格O也同样有效,从而极大地稳定了LLO表面。此外,差分电化学质谱法(DEMS)表明,CrxB 复合物涂层能充分抑制氧的释放,防止过渡金属离子的迁移或溶解,包括允许 Li+ 快速迁移。改性阴极(LLO-CrB)的电压和容量衰减得到了充分抑制,这得益于由平衡的有机/无机成分组成的均匀致密的阴极电解质界面(CEI)。因此,在 1C 下循环 200 次后,LLO-CrB 的比容量为 209.3 mA h g-1(保持率为 95.1%)。这种通过一步湿化学反应实现的双重策略有望应用于其他阴离子氧化还原阴极材料的设计和开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
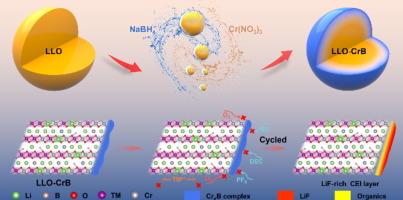
Achievable dual-strategy to stabilize Li-rich layered oxide interface by a one-step wet chemical reaction towards long oxygen redox reversibility
Oxygen release and electrolyte decomposition under high voltage endlessly exacerbate interfacial ramifications and structural degradation of high energy-density Li-rich layered oxide (LLO), leading to voltage and capacity fading. Herein, the dual-strategy of CrxB complex coating and local gradient doping is simultaneously achieved on LLO surface by a one-step wet chemical reaction at room temperature. Density functional theory (DFT) calculations prove that stable B–O and Cr–O bonds through the local gradient doping can significantly reduce the high-energy O 2p states of interfacial lattice O, which is also effective for the near-surface lattice O, thus greatly stabilizing the LLO surface. Besides, differential electrochemical mass spectrometry (DEMS) indicates that the CrxB complex coating can adequately inhibit oxygen release and prevents the migration or dissolution of transition metal ions, including allowing speedy Li+ migration. The voltage and capacity fading of the modified cathode (LLO-CrB) are adequately suppressed, which are benefited from the uniformly dense cathode electrolyte interface (CEI) composed of balanced organic/inorganic composition. Therefore, the specific capacity of LLO-CrB after 200 cycles at 1C is 209.3 mA h g−1 (with a retention rate of 95.1%). This dual-strategy through a one-step wet chemical reaction is expected to be applied in the design and development of other anionic redox cathode materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


