具有钴镍配对位点的硒基异质结用于电催化氧化 5-羟甲基糠醛耦合水分离制氢
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
摘要
5-hydroxymethylfurfural (HMF) 通过电催化氧化为 2,5-呋喃二甲酸 (FDCA),从而产生非典型阴极氢气 (H2),这一点非常吸引人。然而,用于电催化 HMF 氧化反应(e-HMFOR)的电催化剂一直面临着法拉第效率(FE)低和分水电压高的问题。在此,我们提出了一种 NiSeO3@(CoSeO3)4异质结的策略,即构建 Co-Ni 配对位点,其中 Co 位点负责吸附 HMF,而电子则转移到 Ni 位点,从而使 NiSeO3@(CoSeO3)4异质结在 e-HMFOR 和水分离方面具有优异的电催化性能。通过优化条件,NiSeO3@(CoSeO3)4 异质结在 1.3 V 的电压下具有 99.7% 的高转化率、99.9% 的高选择性和 98.4% 的高 FE,同时在 1 M KOH + 0.1 M HMF 的条件下,10 mA cm-2 的电池电压低至 1.31 V。这项研究为 e-HMFOR 以经济的方式实现高附加值 FDCA 耦合水分裂制取 H2 提供了潜在的启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
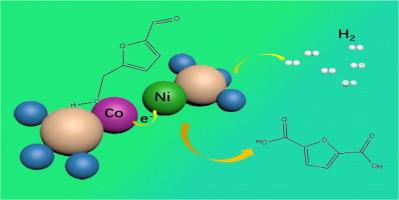
Selenate-based heterojunction with cobalt–nickel paired site for electrocatalytic oxidation of 5-hydroxymethylfurfural coupling water splitting to produce hydrogen
It is very appealing that 5-hydroxymethylfurfural (HMF) is electrocatalytical oxidized as 2,5-furandicarboxylic acid (FDCA) linking to non-classical cathodic hydrogen (H2) production. However, the electrocatalysts for electrocatalytic HMF oxidative reaction (e-HMFOR) have been facing low Faradaic efficiency (FE) and high water splitting voltage. Herein, we propose a strategy of the NiSeO3@(CoSeO3)4 heterojunction by constructing a Co-Ni paired site, where the Co site is in charge of adsorbing for HMF while the electrons are transferred to the Ni site, thus giving the NiSeO3@(CoSeO3)4 heterojunction superior electrocatalytic performances for e-HMFOR and water splitting. By optimizing conditions, the NiSeO3@(CoSeO3)4 heterojunction has high conversion of 99.7%, high selectivity of 99.9%, and high FE of 98.4% at 1.3 V, as well as low cell voltage of 1.31 V at 10 mA cm−2 in 1 M KOH + 0.1 M HMF. This study offers a potential insight for e-HMFOR to high value-added FDCA coupling water splitting to produce H2 in an economical manner.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


