氯化胆碱-尿素深共晶溶剂:二元(表面活性剂)体系的特性、界面行为和协同作用
IF 2
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
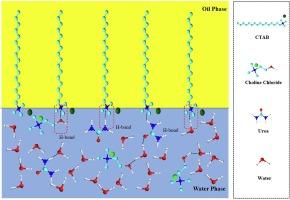
本文重点研究了氯化胆碱:尿素(摩尔比 1:2)DES 的合成和表征。研究了不同浓度的 DES 对低浓度商用表面活性剂(十六烷基三甲基溴化铵)的表面和界面行为的影响。在表面活性剂溶液中加入 DES 后,电泳迁移率和绝对 ZETA 电位值都有所提高,表明表面活性剂在界面上的吸附能力增强。无水滴测量结果表明,二元混合物能够将砂岩的润湿性从中间湿润状态改变为水湿润状态。总之,理化评估显示 DES 能够在低浓度(低于临界胶束浓度)下增强表面活性剂的自组装和润湿行为。在这些溶剂中观察到的现象主要应用于纳米科学、药物输送系统、胶体、催化以及许多其他领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Choline chloride-Urea based deep eutectic Solvent: Characterization, interfacial behavior and Synergism in binary (surfactant) systems
The paper focusses on the synthesis and characterization of choline chloride: urea (molar ratio 1:2) DES. The influence of the varying DES concentration on the surface and interfacial behavior of low-concentration commercial surfactant (cetyl trimethyl ammonium bromide) was examined. The incorporation of DES into the surfactant solution resulted in an augmentation of electrophoretic mobility and absolute zeta potential values, indicative of enhanced surfactant adsorption at the interface. Sessile drop measurements demonstrated the capacity of binary mixture to modify the wettability of sandstone from intermediate-wet state to water-wet condition. In summary, physicochemical evaluation revealed the capability of DES to augment the self-assembly and wetting behavior of surfactant at low concentrations (below critical micelle concentration). The observed phenomenon in these solvents primarily finds utility in nanoscience, drug delivery systems, colloids, catalysis, and applications in many other fields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


