设计 Co/CoO 异质结构,引发原位生成丰富的高价钴物种,以增强硝酸盐到氨的电还原作用
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
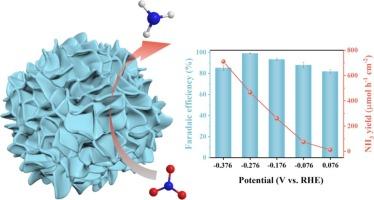
CoO 基材料是电化学硝酸盐还原氨反应(eNO3RR)的理想候选材料。本文采用 Zn/Co 双金属 MOFs 构建 Co/CoO 肖特基异质结构,通过控制 Zn/Co 的比例来设计 Co-CoO 界面。由于 CoO 和 Co 之间的协同效应以及丰富的氧空位,界面优化的 Co/CoO 显示出 713 µmol h-2 cm-2 的 NH3 产率和 99.16 % 的最大法拉第效率。Co-CoO 界面越多,原位生成的高价钴物种(CoOOH)就越多,催化性能就越高。因此,高价钴物种被认为是真正的活性位点。在用作可充电 Zn-NO3- 电池的阴极时,Co/CoO 异质结构的功率密度达到了 4.7 mW cm-2,NH3 产量达到了 131.7 µmol h-2 cm-2,而且工作稳定。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Engineering the Co/CoO heterostructure to trigger the in-situ generation of abundant high-valent cobalt species for enhanced electroreduction of nitrate to ammonia
The CoO-based materials are promising candidates for electrochemical nitrate reduction reaction to ammonia (eNO3RR). Herein, Zn/Co bimetallic MOFs are adopted to construct Co/CoO Schottky heterostructures, where the Co-CoO interfaces are engineered by controlling the Zn/Co ratio. The interface-optimized Co/CoO displays an NH3 yield of 713 µmol h−2 cm−2 and a maximum Faradaic efficiency of 99.16 % due to the synergistic effect between CoO and Co and abundant oxygen vacancies. The more the Co-CoO interfaces, the more the in-situ generated high-valent cobalt species (CoOOH), and the higher the catalytic performance. Therefore, the high-valent cobalt species are considered the true active sites. When used as a cathode in a rechargeable Zn-NO3− battery, the Co/CoO heterostructure achieves a power density of 4.7 mW cm−2 and an NH3 yield of 131.7 µmol h−2 cm−2 with robust working stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


