选择性锂回收的可持续方法:与新型 LMO 流动电极相结合的电容式去离子法
IF 8.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
废弃电池材料的再利用为锂离子(Li+)回收提供了一种环保方法。因此,在本研究中,使用废电池中的锂锰氧化物(LMO)进行流动电极电容去离子(FCDI),从浸出液中选择性地回收 Li+。LMO 的 Li+ 选择性可从含有各种离子物质的废液中有效回收。对 LMO 进行脱硫反应生成的 λ-MnO2 增强了其对 Li+ 的吸附能力。场发射扫描电子显微镜(FE-SEM)和 X 射线衍射(XRD)分析证实了合成的成功,而 BET 分析则验证了该工艺对 Li+ 插层和脱插层的适用性。XPS 分析证实了电极材料中每个阶段都存在 Co2+、Ni2+ 和 Li+,验证了吸附和回收的成功。CV和电化学阻抗谱(EIS)分析表明,与活性炭(AC)相比,λ-MnO2电极具有更低的电荷转移电阻和更高的离子电导率,这表明它具有更优越的电化学性能。基于 λ-MnO2 的 FCDI 系统优于基于 AC 的系统;其去除率高出 3.08 倍,比能量消耗(SEC)低 2.6 倍,平均盐吸附率(ASAR)高出 5.1 倍。吸附和回收实验表明,与 Co2+ 和 Ni2+ 离子相比,Li+ 离子的选择性更高,这进一步凸显了基于 λ-MnO2 的系统的优越性能。总之,从废旧锂离子电池(LIB)中回收的 λ-MnO2 电极不仅是通过 FCDI 工艺进行高效、选择性锂回收的绝佳材料,而且还具有革新可持续锂回收技术的潜力。这项研究在应对气候危机、促进环境保护和保护宝贵资源方面迈出了重要一步。本文章由计算机程序翻译,如有差异,请以英文原文为准。
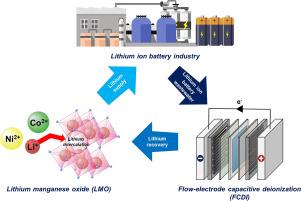
Sustainable approach for selective lithium recovery: Capacitive deionization integrated with novel LMO flow-electrode
Reusing materials from discarded batteries offers an environmentally friendly approach to lithium ion(Li+) recovery. Therefore, in this study, flow-electrode capacitive deionization (FCDI) was employed using lithium manganese oxide (LMO) from spent batteries to selectively recover Li+ from leachate. The Li+ selectivity of LMO enabled effective recovery from waste solutions containing various ionic substances. Delithiation of the LMO to produce λ-MnO2 enhanced its Li+ adsorption capacity. Successful synthesis was confirmed through field-emission scanning electron microscopy (FE-SEM) and X-ray diffraction (XRD) analyses, while BET analysis validated the suitability of the process for Li+ intercalation and deintercalation. XPS analysis confirmed the presence of Co2+, Ni2+, and Li+ in the electrode material at each stage, verifying the successful adsorption and recovery. CV and electrochemical impedance spectroscopy (EIS) analyses showed lower charge transfer resistance and higher ionic conductivity for the λ-MnO2 electrode as compared to activated carbon (AC), indicating its superior electrochemical performance. The λ-MnO2-based FCDI system outperformed the AC-based system; its removal rate was 3.08 times higher, specific energy consumption (SEC) was 2.6 times lower, and average salt adsorption rate (ASAR) was 5.1 times greater. Adsorption and recovery experiments indicated higher selectivity for Li+ ions as compared to Co2+ and Ni2+ ions, further highlighting the superior performance of the λ-MnO2 based system. In conclusion, the λ-MnO2 electrode, recycled from spent lithium-ion batteries (LIBs), is not only an excellent material for high-efficiency and selective lithium recovery via the FCDI process, but also holds the potential to revolutionize sustainable lithium recovery technologies. This study is a significant step towards addressing the climate crisis, promoting environmental protection, and conserving our valuable resources.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


