通过简单置换反应合成的 Ag@Au 核壳纳米粒子修饰的玻璃碳电极用于非酶电化学葡萄糖传感
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
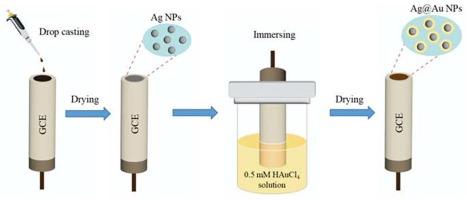
本研究提出了一种用 Ag@Au 合金纳米粒子修饰玻璃碳电极 (GCE) 以提高葡萄糖传感器性能的新策略。合成工艺设计采用简单的置换反应,在银纳米颗粒上生长金层,形成核壳纳米结构。由此获得的纳米复合修饰电极在葡萄糖传感中表现出卓越的电催化活性和稳定性。电化学测量评估了合成的 Ag@Au/GCE 的性能。经 Ag@Au 核壳纳米颗粒修饰的 GCE 在葡萄糖检测方面表现出了卓越的催化活性,从而产生了高电流响应,并且在浓度(10 μM-10 mM)之间具有稳健的线性关系;检测限非常低,仅为 0.04 μM。这种传感器具有线性范围宽、检测限低、选择性高和稳定性强等特点,因此适用于生物样品中葡萄糖的精确定量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ag@Au core–shell nanoparticles modified glassy carbon electrode synthesized by simple displacement reaction for non-enzymatic electrochemical glucose sensing
In this study, a new strategy was presented to enhance glucose sensor performance by modifying a glassy carbon electrode (GCE) with Ag@Au alloy nanoparticles. The synthesis process was designed with a simple replacement reaction to grow the gold layer onto the silver nanparticles to form a core–shell nanostructure. The resulting nanocomposite modified electrode exhibited superior electrocatalytic activity and stability in glucose sensing. Electrochemical measurements were undertaken to assess the performance of the as-synthesized Ag@Au/GCE. The Ag@Au core–shell nanoparticles modified GCE exhibited outstanding catalytic activity towards glucose detection, resulting in a high current response and a robust linear relationship between concentrations (10 μM–10 mM); the detection limit was remarkably low, at 0.04 μM. This sensor demonstrated a wide linear range, low detection limit, and high levels of selectivity and stability, rendering it suitable for accurate glucose quantification in biological samples.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


