华东聊城草酸同源物的混合状态和演化机制:季节和小时观测的启示
IF 4.1
2区 材料科学
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
草酸(C2)是二次有机气溶胶(SOA)的重要示踪剂,但人们对其前体、演化过程和形成机制并不完全了解。这一知识空白导致在评估 SOA 的气候效应和全球预算时存在不确定性。在这里,我们比较了夏季和冬季含 C2 粒子的大小分布、混合分数和演化机制。与冬季相比,夏季 C2 颗粒及其同源物的数量有所减少。然而,C2颗粒占测定颗粒总数的比例却增加了,这表明夏季的颗粒更加老化。夏季气溶胶相对酸度(Rra)较高,原位酸碱度(pHis)较低,表明该季节颗粒的酸性较强。相关性分析和时间变化特征表明,在夏季 9:00 至 15:00 期间,C2 颗粒大多来自较大二羧酸助剂的光化分解,由 O3 浓度驱动。相反,从 16:00 到 20:00,C2 颗粒主要是通过水相氧化形成的,受到较高相对湿度 (RH)、气溶胶液态水含量 (ALWC) 和酸度的影响。此外,重金属颗粒是 C2 颗粒的主要类型,而且 C2 颗粒在夏季与铁的昼夜变化相反,这表明草酸铁复合物的光解是这一时期 C2 颗粒的重要汇。在冬季,生物质燃烧(BB)颗粒最为丰富,左旋葡聚糖与 C2 颗粒之间的强相关性表明 BB 对 C2 颗粒有很大影响。在冬季颗粒状态下,当相对湿度在 40% 到 60% 之间变化时,由酸性驱动的 α-二羰基在水溶液中生成 C2 颗粒的过程最为有效。这些发现突显了 SOA 的来源和演化过程存在小时和季节性变化。在制定控制措施和模拟 SOA 的气候效应时,必须考虑这些变化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
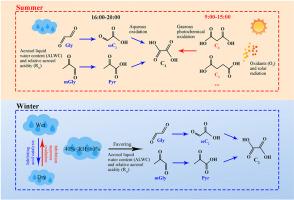
Mixing state and evolutionary mechanism of oxalic acid homologs in Liaocheng, East China: Insights from seasonal and hourly observations
Oxalic acid (C2) is a significant tracer of secondary organic aerosols (SOA), yet its precursors, evolutionary processes, and formation mechanisms are not fully understood. This knowledge gap leads to uncertainties in evaluating the climate effect and global budget of SOA. Here we compared the size distribution, mixing fraction, and evolutionary mechanism of C2-containing particles between summer and winter. In summer, the number of C2 particles and their homologs decreased compared to winter. However, the proportion of C2 relative to the total number of determined particles increased, indicating that the summertime particles are more aged. Higher relative aerosol acidity (Rra) and lower in-situ pH (pHis) in summer suggest that particles are more acidic during this season. Correlation analysis and temporal variation characteristics suggest that from 9: 00 to 15: 00 in summer, C2 particles mostly originate from the photochemical decomposition of larger dicarboxylic aids, driven by O3 concentration. Conversely, from 16: 00 to 20: 00, C2 particles are predominantly formed through aqueous-phase oxidation, influenced by higher relative humidity (RH), aerosol liquid water content (ALWC), and acidity. Additionally, heavy metal particles were the predominant type of C2 particles, and C2 particles exhibited an opposite diurnal variation to Fe in summer, suggesting that the photolysis of iron oxalate complexes is an important sink of C2 particles during this period. In winter, biomass burning (BB) particles were the most abundant, and a robust correlation between levoglucosan and C2 particles indicated a substantial influence of BB on C2 particles. The aqueous generation of C2 particles from α-dicarbonyls driven by acidity was most effective when RH varied from 40% to 60% in the wintertime state of particles. These findings highlight the hourly and seasonal variations in the sources and evolutionary processes of SOA. Such variations must be considered in developing control measures and simulating the climate effect of SOA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Particuology
工程技术-材料科学:综合
CiteScore
6.70
自引率
2.90%
发文量
1730
审稿时长
32 days
期刊介绍:
The word ‘particuology’ was coined to parallel the discipline for the science and technology of particles.
Particuology is an interdisciplinary journal that publishes frontier research articles and critical reviews on the discovery, formulation and engineering of particulate materials, processes and systems. It especially welcomes contributions utilising advanced theoretical, modelling and measurement methods to enable the discovery and creation of new particulate materials, and the manufacturing of functional particulate-based products, such as sensors.
Papers are handled by Thematic Editors who oversee contributions from specific subject fields. These fields are classified into: Particle Synthesis and Modification; Particle Characterization and Measurement; Granular Systems and Bulk Solids Technology; Fluidization and Particle-Fluid Systems; Aerosols; and Applications of Particle Technology.
Key topics concerning the creation and processing of particulates include:
-Modelling and simulation of particle formation, collective behaviour of particles and systems for particle production over a broad spectrum of length scales
-Mining of experimental data for particle synthesis and surface properties to facilitate the creation of new materials and processes
-Particle design and preparation including controlled response and sensing functionalities in formation, delivery systems and biological systems, etc.
-Experimental and computational methods for visualization and analysis of particulate system.
These topics are broadly relevant to the production of materials, pharmaceuticals and food, and to the conversion of energy resources to fuels and protection of the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


