丙酮和异丙醇交叉对直接异丙醇燃料电池的影响
IF 4.7
3区 工程技术
Q2 ELECTROCHEMISTRY
引用次数: 0
摘要
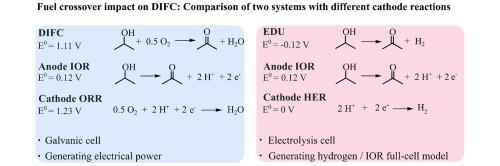
液态有机氢载体(LOHC)为在现有基础设施内按需储存和释放氢气提供了一种前景广阔的选择。直接异丙醇燃料电池(DIFC)使用电化学丙酮/异丙醇液态有机氢载体偶联物,将环境条件下的高燃料能量密度与无二氧化碳直接发电的优势结合在一起。与其他酒精燃料电池一样,DIFC 结合了两个动力学速度较慢的反应,即异丙醇氧化反应(IOR)和氧还原反应(ORR),需要相当大的过电位来驱动反应。因此,很难对整个电池的动力学特性进行分解。因此,这项研究采用了电解电化学脱氢单元(EDU),由酸性介质中的 IOR 和动力学快速氢进化反应组成。然后,该 EDU 可作为 IOR 全电池模型来深入了解 DIFC。相应地,展示的工作是对内部制造的催化剂涂层膜电极组件作为氢燃料电池、DIFC 和 EDU 进行比较研究。它研究了 DIFC 的特征,并展示了丙酮和异丙醇交叉如何影响 DIFC 的阴极。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The influence of acetone and isopropanol crossover on the direct isopropanol fuel cell
Liquid organic hydrogen carriers (LOHC) offer a promising option to store and release hydrogen on demand within existing infrastructure. The direct isopropanol fuel cell (DIFC) uses the electrochemical acetone/isopropanol LOHC couple and combines the advantages of high fuel energy density at ambient conditions with CO2-free direct electricity production. Like other alcohol fuel cells, the DIFC combines two kinetically slow reactions, the isopropanol oxidation reaction (IOR) and the oxygen reduction reaction (ORR), requiring considerable overpotentials to drive the reactions. Accordingly, deconvoluting kinetic characteristics in the full cell is difficult. Therefore, this work uses the electrolytic electrochemical dehydrogenation unit (EDU), consisting of the IOR and the kinetically fast hydrogen evolution reaction in acidic media. This EDU then serves as an IOR full-cell model to get insights on the DIFC. Correspondingly, the demonstrated work is a comparison study investigating in-house fabricated catalyst-coated membrane electrode assemblies as hydrogen fuel cells, DIFC, and EDU. It investigates characteristic features of the DIFC and demonstrates how the acetone and isopropanol crossover affect the cathode of the DIFC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochemistry Communications
工程技术-电化学
CiteScore
8.50
自引率
3.70%
发文量
160
审稿时长
1.2 months
期刊介绍:
Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication. Short communications are limited to a maximum of 20,000 characters (including spaces) while full communications and mini reviews are limited to 25,000 characters (including spaces). Supplementary information is permitted for full communications and mini reviews but not for short communications. We aim to be the fastest journal in electrochemistry for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


