迷走神经刺激:通过减少细胞因子(白细胞介素-6)、减弱 SASP、增强肿瘤免疫力来治疗胶质母细胞瘤和实体瘤的新概念
IF 3.7
Q2 IMMUNOLOGY
引用次数: 0
摘要
免疫肿瘤学,特别是免疫检查点抑制剂(ICIs),给癌症治疗带来了革命性的变化,长期疗效显著,生存率提高,包括脑转移癌患者。胶质母细胞瘤和其他原发性脑肿瘤对 ICIs 单药治疗或与标准疗法联用均难治。肿瘤微环境(TME)构成了多种生物学障碍:血脑屏障、免疫抑制、异质性和肿瘤浸润。对衰老相关分泌表型(SASP)和胶质瘤临床前模型的基因组分析表明,一种令人兴奋的方法是对胶质瘤微环境进行重编程,减少 SASP 的促炎症、促肿瘤细胞因子,尤其是白细胞介素-6(IL-6)。现在提出的一个可检验的假设是,通过利用人体的 "炎症反射 "来减少细胞因子,从而调节免疫系统。迷走神经刺激可通过胆碱能、α7 尼古丁乙酰胆碱受体激动剂(α7nAchR)激活 T 细胞免疫,并在全身抑制 IL-6,以及 SASP 的其他促炎细胞因子、白细胞介素-1β(IL-1β)和肿瘤坏死因子-α(TNF-α)。该假说预测,电激活迷走神经,减少细胞因子,结合 ICIs,可将免疫抗性("冷")肿瘤转化为免疫反应性("热")肿瘤,并阻止胶质瘤的发展。该假说还将癌症视为一种免疫 "自律神经失调",通过迷走神经刺激(VNS)治疗干预,可重置全身和局部细胞因子水平。为证实这一假设,下一步理应进行前瞻性、随机化的 II 期临床试验。由于新兴的 "癌症神经科学 "领域显示了神经对多种肿瘤类型的调控作用,通过 VNS 减少细胞因子对其他形式的人类癌症(如乳腺癌、结直肠癌、头颈癌、肺癌、黑色素瘤、卵巢癌、胰腺癌和前列腺癌)也有帮助。由于 IL-6 和其他促炎细胞因子参与了癌症的发生、发展、扩散和复发,微创 VNS 可用于抑制胶质瘤或癌症的发展,同时还能减轻抑郁和/或癫痫发作,从而提高生活质量。目前的假设将胶质瘤的病理生理学重新想象为自主神经功能紊乱,其治疗目标是重置自主神经系统并形成免疫反应状态,以阻止肿瘤进展并防止复发。VNS 作为一种控制癌症的新方法,可与 ICIs、标准疗法或临床试验中的新兴免疫疗法(树突状细胞、mRNA 或嵌合抗原受体 (CAR) T 细胞疫苗)结合使用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
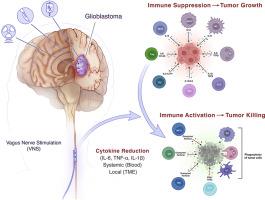
Vagus nerve stimulation: Novel concept for the treatment of glioblastoma and solid cancers by cytokine (interleukin-6) reduction, attenuating the SASP, enhancing tumor immunity
Immuno-oncology, specifically immune checkpoint inhibitors (ICIs), has revolutionized cancer care with dramatic, long-term responses and increased survival, including patients with metastatic cancer to the brain. Glioblastomas, and other primary brain tumors, are refractory to ICIs as monotherapy or in combination with standard therapy. The tumor microenvironment (TME) poses multiple biological hurdles: blood-brain barrier, immune suppression, heterogeneity, and tumor infiltration. Genomic analysis of the senescence-associated secretory phenotype (SASP) and preclinical models of glioma suggest that an exciting approach would entail reprogramming of the glioma microenvironment, attenuating the pro-inflammatory, pro-tumorigenic cytokines of the SASP, especially interleukin-6 (IL-6). A testable hypothesis now proposed is to modulate the immune system by harnessing the body's ‘inflammatory reflex’ to reduce cytokines. Vagus nerve stimulation can activate T cell immunity by the cholinergic, α7nicotinic acetylcholine receptor agonist (α7nAchR), and suppress IL-6 systemically, as well as other pro-inflammatory cytokines of the SASP, interleukin -1β (IL-1β) and tumor necrosis factor-alpha (TNF-α). The hypothesis predicts that electrical activation of the vagus nerve, with cytokine reduction, in combination with ICIs, would convert an immune resistant (“cold”) tumor to an immune responsive (“hot”) tumor, and halt glioma progression. The hypothesis also envisions cancer as an immune “dysautonomia” whereby a therapeutic intervention, vagus nerve stimulation (VNS), resets the systemic and local cytokine levels. A prospective, randomized, phase II clinical trial, to confirm the hypothesis, is a logical, exigent, next step. Cytokine reduction by VNS could also be useful for other forms of human cancer, e.g., breast, colorectal, head and neck, lung, melanoma, ovarian, pancreatic, and prostate cancer, as the emerging field of “cancer neuroscience” shows a role for neural regulation of multiple tumor types. Because IL-6, and companion pro-inflammatory cytokines, participate in the initiation, progression, spread and recurrence of cancer, minimally invasive VNS could be employed to suppress glioma or cancer progression, while also mitigating depression and/or seizures, thereby enhancing quality of life. The current hypothesis reimagines glioma pathophysiology as a dysautonomia with the therapeutic objective to reset the autonomic nervous system and form an immune responsive state to halt tumor progression and prevent recurrence. VNS, as a novel method to control cancer, can be administered with ICIs, standard therapy, or in clinical trials, combined with emerging immunotherapy: dendritic cell, mRNA, or chimeric antigen receptor (CAR) T cell vaccines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Brain, behavior, & immunity - health
Biological Psychiatry, Behavioral Neuroscience
CiteScore
8.50
自引率
0.00%
发文量
0
审稿时长
97 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


