肌成纤维细胞通过免疫特权机制持续介导口腔黏膜下纤维化:揭示发病机制
Q1 Medicine
Journal of oral biology and craniofacial research
Pub Date : 2024-10-20
DOI:10.1016/j.jobcr.2024.10.008
引用次数: 0
摘要
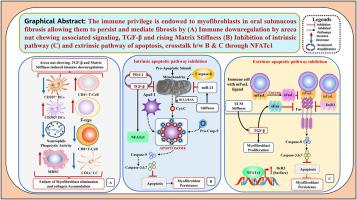
免疫特权是指在不引起炎症免疫反应的情况下耐受外来抗原的能力。一种结构的免疫特权状态受先天性免疫反应和适应性免疫反应的调节,有多种机制可以解释这种状态。在口腔黏膜下纤维化(OSF)的背景下,肌成纤维细胞通过获得免疫特权表型使纤维化永久化的作用正在不断发展。肌成纤维细胞通过 Fas/FasL 自分泌途径持续存在,并诱导上皮细胞凋亡,这就是纤维化区域凋亡细胞并存的原因。然而,基质硬度的增加除了激活 TGF-β 外,还降低了肌成纤维细胞中 Fas 的表面表达,增加了它们对细胞凋亡的抵抗力。免疫检查点蛋白程序性死亡配体 1(PD-L1)和 TGF-β 之间的相互放大循环涉及 YAP-TAZ 和 SMAD2,3 通路,并显著增强了促组织坏死的信号传导。基质硬度的增加也会增强 cMYC 的表达,进而放大肌成纤维细胞上的 PD-L1 水平。肌成纤维细胞上 PD-L1 的增加通过作用于 T 细胞表面的程序性细胞死亡蛋白 1(PD-1)受体,对归巢到纤维化区域的 CD4+ T 细胞的表型进行微观设计,将这些细胞从抗纤维化细胞转化为产生 IL-17A 和 TGF-β 的促纤维化细胞。这篇手稿从机理上揭示了肌成纤维细胞如何通过逃避免疫系统来避免OSF中的细胞凋亡。针对具有FAS-FASL通路依赖性特征的肌成纤维细胞的免疫特权表型是逆转OSF的理想策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Myofibroblasts persist through immune privilege mechanisms to mediate oral submucous fibrosis: Uncovering the pathogenesis
Immune privilege is the ability to tolerate foreign antigens without eliciting an inflammatory immune response. Several mechanisms explain a structure's immune privilege status, which is regulated by innate and adaptive immune responses. The role of myofibroblasts in perpetuating fibrosis by acquiring an immune privileged phenotype against the backdrop of oral submucous fibrosis (OSF) is evolving. Myofibroblasts persist through the Fas/FasL autocrine pathway and induce apoptosis in epithelial cells, explaining the juxtaposition of apoptotic cells in areas of fibrosis. However, increased matrix stiffness, in addition to activating TGF-β, reduces Fas surface expression in myofibroblasts, increasing their resistance to apoptosis. The reciprocal amplification loop between the immune checkpoint proteins programmed death-ligand 1 (PD-L1) and TGF-β involves the YAP-TAZ and SMAD2,3 pathways and dramatically enhances profibrotic signalling. Increased matrix stiffness also enhances cMYC expression, which subsequently amplifies PD-L1 levels on myofibroblasts. The increase in PD-L1 on the myofibroblast microengineers the phenotype of CD4+ T cells homing to fibrotic areas by acting on the programmed cell death protein 1 (PD-1) receptor on the T-cell surface, converting these cells from antifibrotic cells to profibrotic cells that produce IL-17A and TGF-β. This manuscript provides mechanistic insight into how myofibroblasts avoid apoptosis in OSFs by evading the immune system. Targeting an immune-privileged phenotype in myofibroblasts with FAS-FASL pathway-dependent characteristics is an ideal strategy for reversing OSF.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of oral biology and craniofacial research
Medicine-Otorhinolaryngology
CiteScore
4.90
自引率
0.00%
发文量
133
审稿时长
167 days
期刊介绍:
Journal of Oral Biology and Craniofacial Research (JOBCR)is the official journal of the Craniofacial Research Foundation (CRF). The journal aims to provide a common platform for both clinical and translational research and to promote interdisciplinary sciences in craniofacial region. JOBCR publishes content that includes diseases, injuries and defects in the head, neck, face, jaws and the hard and soft tissues of the mouth and jaws and face region; diagnosis and medical management of diseases specific to the orofacial tissues and of oral manifestations of systemic diseases; studies on identifying populations at risk of oral disease or in need of specific care, and comparing regional, environmental, social, and access similarities and differences in dental care between populations; diseases of the mouth and related structures like salivary glands, temporomandibular joints, facial muscles and perioral skin; biomedical engineering, tissue engineering and stem cells. The journal publishes reviews, commentaries, peer-reviewed original research articles, short communication, and case reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


