物理和化学改性沸石模板化氮碳增强氢气吸附能力
IF 6.2
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
碳材料具有显著的比表面积、独特的孔径特征以及与金属或非金属功能化的能力,因此在吸附氢气方面具有巨大的潜力。在这项工作中,通过改变制备条件对沸石模板碳进行了物理和化学修饰,以研究其对结构和氢吸附能力的影响。根据合成条件的不同,模板碳的比表面积在 608-1665 m2/g 之间,孔体积在 0.63-1.00 cc/g 之间,微孔率为 28-48%。表面积和孔体积随着碳沉积温度(650 至 750 °C)的升高而增大,而在碳沉积温度较高的 850 °C,表面积和孔体积均有所减小。在热处理温度为 900 ℃ 时,模板碳的表面积和孔隙率都较高。在碳沉积过程中,碳基体中氮杂质原子的掺入可能有助于提高孔隙率。使用氩气作为载气可获得最高的表面积(1665 m2/g)、微孔面积(597 m2/g)和孔隙率(1.0 cc/g)。同样的模板碳在 15 巴压力下,温度分别为 25 和 -196 ℃ 时的最大氢吸附容量分别为 0.20 和 2.81 wt%。在模板碳中加入铂后,氢气吸附能力显著提高,25 °C时从0.20 wt%提高到0.28 wt%,-196 °C时从2.81 wt%提高到3.24 wt%。铂对氢的强亲和力可能增强了对氢的吸附。本文章由计算机程序翻译,如有差异,请以英文原文为准。
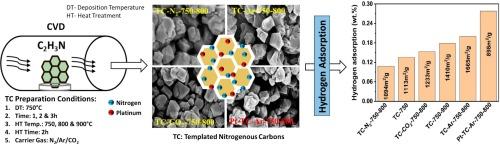
Physically and chemically modified zeolite templated nitrogenous carbons for enhanced hydrogen adsorption
Carbon materials have great potential for hydrogen adsorption due to their remarkable specific surface area, unique pore size characteristics and ability to functionalize with metal or non-metal. In this work, zeolite templated carbons were physically and chemically modified by varying preparation conditions to study their impact on structure and hydrogen adsorption capacity. The resultant templated carbons showed surface area in the range of 608–1665 m2/g and pore volume between 0.63 to 1.00 cc/g, with 28–48 % microporosity depending on synthesis conditions. The surface area and pore volume increased with increasing carbon deposition temperature from 650 to 750 °C and both decreased at higher carbon deposition temperature of 850 °C. At heat treatment temperature of 900 °C, the surface area and pore volume of templated carbons were observed to be higher. Incorporation of nitrogen heteroatom in carbon matrix during carbon deposition might have facilitated porosity. Use of argon as carrier gas resulted in the highest surface area (1665 m2/g), micropore area (597 m2/g) and pore volume (1.0 cc/g). The same templated carbon showed maximum hydrogen adsorption capacity of 0.20 and 2.81 wt% at 25 and –196 °C, respectively at 15 bar. On addition of platinum to templated carbon, the hydrogen adsorption capacity was significantly improved from 0.20 to 0.28 wt% at 25 °C and from 2.81 to 3.24 wt% at –196 °C. The strong affinity of Pt for hydrogen might have enhanced hydrogen adsorption.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

FlatChem
Multiple-
CiteScore
8.40
自引率
6.50%
发文量
104
审稿时长
26 days
期刊介绍:
FlatChem - Chemistry of Flat Materials, a new voice in the community, publishes original and significant, cutting-edge research related to the chemistry of graphene and related 2D & layered materials. The overall aim of the journal is to combine the chemistry and applications of these materials, where the submission of communications, full papers, and concepts should contain chemistry in a materials context, which can be both experimental and/or theoretical. In addition to original research articles, FlatChem also offers reviews, minireviews, highlights and perspectives on the future of this research area with the scientific leaders in fields related to Flat Materials. Topics of interest include, but are not limited to, the following: -Design, synthesis, applications and investigation of graphene, graphene related materials and other 2D & layered materials (for example Silicene, Germanene, Phosphorene, MXenes, Boron nitride, Transition metal dichalcogenides) -Characterization of these materials using all forms of spectroscopy and microscopy techniques -Chemical modification or functionalization and dispersion of these materials, as well as interactions with other materials -Exploring the surface chemistry of these materials for applications in: Sensors or detectors in electrochemical/Lab on a Chip devices, Composite materials, Membranes, Environment technology, Catalysis for energy storage and conversion (for example fuel cells, supercapacitors, batteries, hydrogen storage), Biomedical technology (drug delivery, biosensing, bioimaging)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


