在低分压范围内通过溶胶-凝胶合成在离子液体-二氧化硅纳米复合材料中实现表面增强型二氧化碳捕获
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
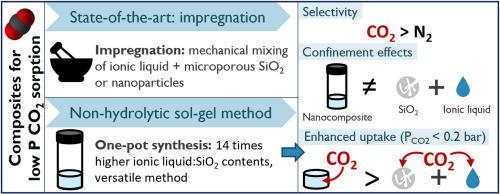
含离子液体的二氧化硅纳米复合材料可从含氮、氧和甲烷的混合气体中捕获二氧化碳。此类纳米复合材料的合成方法包括浸渍多孔二氧化硅和通过溶胶-凝胶工艺进行单锅合成。本研究探讨了一种非水解溶胶-凝胶路线,用于在低分压(0.1-0.4 巴)下捕获二氧化碳的纳米复合材料。正硅酸四乙酯(TEOS)前驱体凝结后,在离子液体畴周围形成二氧化硅基质、这种离子液体以双(三氟磺酰亚胺)(TFSI-)为基础,含有 1-丁基-1-甲基吡咯烷鎓(BMP+)、1-丁基-3-甲基咪唑鎓(BMI+)、1-乙基-3-甲基咪唑鎓(EMI+)和 1-己基-3-甲基咪唑鎓(HMI+)阳离子。采用一锅合成法可以探索这种纳米复合材料的二氧化碳吸附性,其离子液体与二氧化硅的含量比以前报道的研究高出 14 倍。此外,所选的合成方法为沉积方法及其控制提供了更大的可调性。在溶剂萃取和超临界干燥材料以去除离子液体后,在 77 K 下通过 N2 吸附等温线对二氧化硅主基质进行了表征。通过扫描电子显微镜(SEM)成像观察了独立二氧化硅网络的孔径分布,并用巴雷特-乔伊纳-哈伦达(BJH)法评估了不同[BMP][TFSI]-二氧化硅比的纳米复合材料。根据 303 K 下的二氧化碳吸附等温线评估了低至 0.1 bar 压力下的二氧化碳吸收率。[BMP][TFSI]的封闭性对较低分压下的二氧化碳吸收率产生了有利影响,其吸收率比所含离子液体和二氧化硅的预期吸收率之和高出五倍。报告结果表明,单锅合成法的优势在于纳米复合材料的含量和应用具有更广泛的可调性,而且与纳米复合材料的单个成分相比,在较低分压下性能更高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Surface-enhanced CO2 capture in ionic liquid-silica nanocomposites via sol-gel synthesis in the low partial pressure range
Ionic liquid-containing silica nanocomposites enable the capture of carbon dioxide from gas mixtures containing nitrogen, oxygen, and methane. Synthesis methods explored for such nanocomposites include impregnating porous silica and one-pot synthesis via a sol-gel process. This research investigates a non-hydrolytic sol-gel route for nanocomposite materials enabling CO2 capture at low partial pressures (0.1–0.4 bar). The tetraethyl orthosilicate (TEOS) precursor condensation resulted in a silica matrix formed around ionic liquid domains, for bis(trifluorosulfonylimide) (TFSI−)-based ionic liquids with 1-butyl-1-methylpyrrolidinium (BMP+), 1-butyl-3-methylimidazolium (BMI+), 1-ethyl-3-methylimidazolium (EMI+) and 1-hexyl-3-methylimidazolium (HMI+) cations. Using a one-pot synthesis method enables exploring CO2 sorption in such nanocomposites for ionic liquid-to-silica contents up to fourteen times higher than in previously reported studies. Moreover, the selected synthesis method provides greater tunability in deposition methods and their control. The silica host matrix was characterized by N2 adsorption isotherms at 77 K after solvent extraction and supercritical drying of the material for ionic liquid removal. The pore size distribution of the freestanding silica network was observed via Scanning Electron Microscope (SEM) imaging and assessed with the Barrett-Joyner-Halenda (BJH) method for nanocomposites of different [BMP][TFSI]-to-silica ratio. The CO2 uptake at pressures down to 0.1 bar was evaluated from CO2 adsorption isotherms at 303 K. The confinement of [BMP][TFSI] resulted in a beneficial effect for the CO2 uptake at lower partial pressures, with an uptake five times higher than the sum of the individual uptake expected from the contained ionic liquid and silica. The reported results show the advantage of a one-pot synthesis method for broader tunability of the nanocomposite, both regarding its content and application, as well as increased performance at lower partial pressures compared to the nanocomposite's individual constituents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


