对掺钴 g-C3N4 活化 PMS 有效去除环丙沙星的动力学模型的新认识:双指数衰减模型与机理
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
本研究采用高温煅烧法合成了掺钴氮化石墨碳(Co-CN),并将其用于活化过硫酸盐(PMS)以去除环丙沙星(CIP)。实验结果表明,在 0.5 g/L 0.25Co-CN、2 mM PMS、初始溶液 pH 值为 7、初始 CIP 浓度为 5 mg/L、温度为 30 ℃ 的优化条件下,45 分钟内 CIP 的去除率可达 99.5%。Co-CN 活化 PMS 去除 CIP 的动力学可通过双指数衰减函数精确建模,表明 CIP 去除过程包括快速反应阶段和慢速反应阶段。此外,经过五个循环的重复使用,催化剂对 CIP 的去除率保持在 83.3% 的可观水平,反应前后的表征证实了 0.25Co-CN 卓越的重复使用性和稳定性。淬火实验和电子顺磁共振(EPR)测试证实了 1O2 在催化降解机制中的主要作用。研究发现,0.25Co-CN 中的 CoN4 活性位点对 CIP 的吸附和后续降解至关重要。本研究提出了一种新的动力学模型,用于拟合吸附和催化协同作用下的 CIP 去除过程,为理解有机污染物去除的动态过程提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
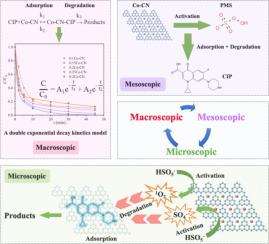
New insight to kinetics model for cobalt doped g-C3N4 activated PMS for the efficient removal of ciprofloxacin: A bi-exponential decay model and mechanism
In this work, cobalt-doped graphitic carbon nitride (Co-CN) was synthesized through a high-temperature calcination method and employed to activate peroxymonosulfate (PMS) for ciprofloxacin (CIP) removal. The experimental outcomes demonstrated that under optimized conditions of 0.5 g/L 0.25Co-CN, 2 mM PMS, an initial solution pH of 7, an initial CIP concentration of 5 mg/L, and at a temperature of 30 °C, the removal efficiency of CIP can reach 99.5 % within 45 min. The kinetics of CIP removal by Co-CN activated PMS are accurately modeled by a bi-exponential decay function, indicating a CIP removal process involving both rapid and slower reaction phases. Furthermore, after five cycles of reuse, the catalyst maintained a respectable removal efficiency of 83.3 % for CIP, with pre- and post-reaction characterizations affirming the excellent reusability and stability of 0.25Co-CN. Quenching experiments and Electron Paramagnetic Resonance (EPR) tests confirmed the predominance role of 1O2 in the catalytic degradation mechanism. The Co![]() N4 active sites within 0.25Co-CN were identified as crucial for adsorption and subsequent degradation of CIP. In this study, a novel kinetics model is proposed for fitting the CIP removal process under the synergistic effect of adsorption and catalysis, which provides new insights into the understanding of the dynamic process of organic pollutant removal.
N4 active sites within 0.25Co-CN were identified as crucial for adsorption and subsequent degradation of CIP. In this study, a novel kinetics model is proposed for fitting the CIP removal process under the synergistic effect of adsorption and catalysis, which provides new insights into the understanding of the dynamic process of organic pollutant removal.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


