在海水电解质中用于高效甘油氧化和氢气进化的自支撑铁钴镍铜高熵合金纳米片阵列†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
开发用于海水电解质中氢气进化和甘油氧化的高效双功能电催化剂对于生产高附加值化学品具有重要意义。在此,通过一种简单的电沉积方法,在碳布基底上成功合成了自支撑的铁钴镍铜磷(FeCoNiCuP)高熵合金纳米片阵列(HEANAs)。所制备的催化剂在甘油氧化反应(GOR)中表现出卓越的性能,在 10 mA cm-2 的条件下电位低至 1.28 V,甲酸法拉第效率(FE)高达 87.9%。此外,这种催化剂还具有优异的氢进化反应(HER)性能。理论计算表明,FeCoNiCuP 的铁桥位和 CuNiFe 桥位分别是 GOR 和 HER 的活性中心。在 GOR 辅助电解槽中使用 FeCoNiCuP HEANAs,可以在 10 mA cm-2 的条件下大幅降低电位至 1.40 V,在阳极产生甲酸盐,在阴极产生氢气,并且具有高 FE 和出色的稳定性。此外,该催化剂在海水电解液中也能保持稳定的性能,不会干扰氯的演化,为可持续和环保型高价值化学品生产提供了一条前景广阔的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
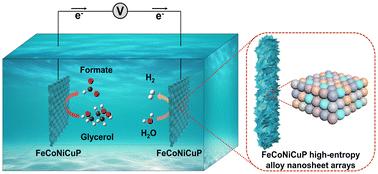
Self-supported FeCoNiCuP high-entropy alloy nanosheet arrays for efficient glycerol oxidation and hydrogen evolution in seawater electrolytes†
The development of highly efficient and bifunctional electrocatalysts for hydrogen evolution and glycerol oxidation in seawater electrolytes is of great significance for the production of high value-added chemicals. Herein, self-supported FeCoNiCuP high entropy alloy nanosheet arrays (HEANAs) were successfully synthesized on a carbon cloth substrate through a straightforward electrodeposition method. The resulting catalyst demonstrates remarkable performance towards glycerol oxidation reaction (GOR) with a low potential of 1.28 V at 10 mA cm−2 and an impressive formate Faradaic efficiency (FE) of 87.9%. Additionally, this catalyst exhibits excellent hydrogen evolution reaction (HER) performance. Theoretical calculations identify Fe and CuNiFe bridge sites of FeCoNiCuP as active centers for GOR and HER, respectively. Utilizing FeCoNiCuP HEANAs in the GOR-assisted electrolyzer achieves a substantially reduced potential of 1.40 V at 10 mA cm−2, producing formate at the anode and hydrogen at the cathode with high FE and outstanding stability. Furthermore, the catalyst maintains consistent performances in seawater electrolyte without interfering with chlorine evolution, offering a promising avenue for sustainable and environmentally friendly high-value chemical production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


