大小可调的纳米团簇可重塑肿瘤和肿瘤引流淋巴结的免疫抑制微环境,从而改善癌症免疫疗法。
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
在过去几年中,通过免疫调节剂重塑免疫抑制性肿瘤微环境(TME)的研究已经取得了很大进展。然而,能够同时调节免疫抑制性肿瘤微环境和肿瘤引流淋巴结(TDLNs)的策略仍处于起步阶段。在这里,我们报告了一种对 pH 值敏感的尺寸可转换纳米团簇 SPN-R848,它能同时在肿瘤和肿瘤引流淋巴结中积聚以激活免疫。SPN-R848由瑞喹莫德(R848)-共轭聚氨基胺(PAMAM)衍生物自组装而成,原始尺寸约为150纳米。尺寸的缩小不仅增强了它们在原发肿瘤中的聚集和灌注,还促进了它们在 TDLNs 中的转运和分布。因此,SPN-R848通过将TDLNs中的M2巨噬细胞极化为M1巨噬细胞和活化树突状细胞(DCs),显著重塑了免疫抑制TME,协同促进了细胞毒性T细胞的产生和刺激,抑制了乳腺癌和黑色素瘤小鼠模型的肿瘤生长。我们的研究表明,在肿瘤和TDLNs中共同激活免疫微环境可能是激发强大抗肿瘤免疫力的一个有前途的方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
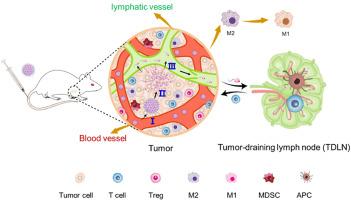
A size-switchable nanocluster remodels the immunosuppressive microenvironment of tumor and tumor-draining lymph nodes for improved cancer immunotherapy
Remodeling the immunosuppressive tumor microenvironment (TME) by immunomodulators has been well studied in the past years. However, strategies that enable concurrent modulation of both the immunosuppressive TME and tumor-draining lymph nodes (TDLNs) are still in the infancy. Here, we report a pH-sensitive size-switchable nanocluster, SPN-R848, to achieve simultaneous accumulation in tumor and TDLNs for immune activation. SPN-R848 with original size around 150 nm was formed by self-assembly of resiquimod (R848)-conjugated polyamidoamine (PAMAM) derivative, which could disintegrate into its small constituents (~ 8 nm) upon exposure to tumor acidity. The size reduction not only enhanced their accumulation and perfusion in the primary tumor, but promoted their transport and distribution in TDLNs. Accordingly, SPN-R848 remarkably remodeled the immunosuppressive TME by polarizing M2 to M1 macrophages and activated dendritic cells (DCs) in TDLNs, which synergistically facilitated the production and stimulation of cytotoxic T cells, and inhibited tumor growth in breast cancer and melanoma mouse models. Our study suggests that co-activation of immune microenvironments in both tumor and TDLNs may represent a promising direction to elicit strong antitumor immunity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


