用于手性高效液相色谱分离的手性微孔有机网络的 "点击 "后合成技术
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
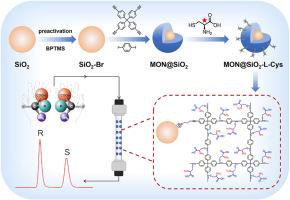
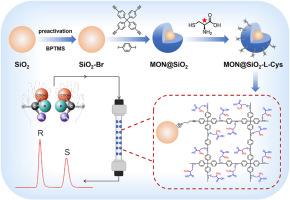
背景手性分离具有重要意义,但在分析化学中仍具有挑战性。基于手性固定相的高效液相色谱(HPLC)被认为是最有效、应用最广泛的手性分离方法之一。微孔有机网络(MONs)是一类新兴的多孔材料,具有可设计的拓扑结构、较大的比表面积、丰富的微孔和优异的稳定性,在色谱分离领域引起了极大的兴趣。结果我们首次将手性 MON@SiO2-L-Cys 作为新型固定相用于手性 HPLC。MON@SiO2-L-Cys是通过特定的硫醇-炔点击反应与L-半胱氨酸(L-Cys)进行后合成的。经填充的 MON@SiO2-L-Cys 色谱柱能够分离手性芳香醇、酯和腈,并具有良好的分辨率(2-苯基-2-戊醇为 4.00)和高选择性(盐酸普萘洛尔为 3.56)。研究了样品用量、流动相组成和分离温度的影响。MON@SiO2-L-Cys 填料色谱柱还与三种商用手性色谱柱具有手性识别互补性。例如,2-氨基-2-苯乙醇和盐酸普萘洛尔可在MON@SiO2-L-Cys填料柱上实现基线分离,但在商用Chiralpak IA、IB和IH柱上却无法分离。此外,MON@SiO2-L-Cys填料柱在使用9个月后仍能为2-苯基-2-戊醇提供相当的分离性能。 重要意义 本研究首次报道了通过简便的点击后合成策略构建手性MON基固定相用于手性高效液相色谱分离的实例。该研究表明,手性 MONs 作为高效手性固定相用于手性 HPLC 具有广阔的前景,并可能为 MONs 在手性分离领域的应用开辟一个新的领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
“Click” post-synthesis of chiral microporous organic network for chiral high-performance liquid chromatographic separation
Background
Chiral separation is of great significance but remains challenging in analytical chemistry. Chiral stationary phase based high-performance liquid chromatography (HPLC) is regarded as one of the most effective and widely used methods for chiral resolution. Microporous organic networks (MONs) are an emerging class of porous materials with designable topology, large surface area, abundant micropores, and excellent stabilities that have attracted tremendous interests in chromatographic separation. However, the usage of chiral MONs as chiral stationary phases for chiral HPLC separation has not been reported so far.
Results
Herein, we present the first example of chiral MON@SiO2-L-Cys as a novel stationary phase for chiral HPLC. MON@SiO2-L-Cys is post-synthesized with l-cysteine (L-Cys) via the specific thiol-yne click reaction. The packed MON@SiO2-L-Cys column is able to separate chiral aromatic alcohols, esters, and nitriles with good resolution (4.00 for 2-phenyl-2-pentanol) and high selectivity (3.56 for propranolol hydrochloride). Effects of sample dosage, mobile phase composition, and separation temperature are studied. The MON@SiO2-L-Cys packed column also exhibits chiral recognition complementarity with three commercial chiral columns. For example, 2-amino-2-phenylethanol and propranolol hydrochloride are able to achieve baseline separation on MON@SiO2-L-Cys packed column, but cannot be separated on commercial Chiralpak IA, IB, and IH columns. In addition, the MON@SiO2-L-Cys packed column gives comparable separation performance for 2-phenyl-2-pentanol even after 9 months’ utilization.
Significance
This work reports the first example of constructing chiral MON-based stationary phase for chiral HPLC separation via the facile click post-synthesis strategy. This study demonstrates the considerable prospect of chiral MONs as efficient chiral stationary phases for chiral HPLC and may open up a new area of MONs in chiral separation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


