通过碳量子点电子桥在 BiOBr/Ti3C2 上强化内置电场和介导肖特基势垒高度,实现高效光催化喹诺酮类抗生素降解
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
由金属-半导体接触形成的基于肖特基结的光催化剂很有吸引力,但其光催化性能却因内置电场(IEF)驱动力差和肖特基势垒高度(SBH)过高而受到限制。高效电荷转移的一种前瞻性策略是通过插入可行的缓冲层来调节界面间隙态。在这里,碳量子点(CQDs)被插入到 BiOBr/Ti3C2 肖特基异质结中,通过 Bi-O-C 和 Ti-O-C 化学键形成电子桥。CQDs 电子桥调节了电荷的空间分布,使 IEF 提高了 3.4 倍,并促进了 BiOBr/CQDs/Ti3C2 内电荷的有效分离和转移。BiOBr/CQDs/Ti3C2 的载流子寿命延长至 2357.8 ps,有效提高了电荷载流子密度。此外,Ti-C-O 和 Bi-C-O 键加强了界面相互作用,使 BiOBr/CQDs/Ti3C2 中的 SBH 从 2.02 eV 显著降低到 1.77 eV,加速了电荷在金属-半导体界面上的传输。值得注意的是,BiOBr/CQDs/Ti3C2 对多种喹诺酮类抗生素(FQs)具有优异的光催化降解能力,尤其是在 120 分钟内对莫西沙星(MOX,96.1%)的光催化降解能力分别是 BiOBr、BiOBr/CQDs 和 BiOBr/Ti3C2 的 2.63、2.48 和 1.84 倍。此外,BiOBr/CQDs/Ti3C2 还具有较高的环境适应性和循环稳定性。这项工作为在肖特基光催化剂中构建电子桥以提高光催化活性提供了一种新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
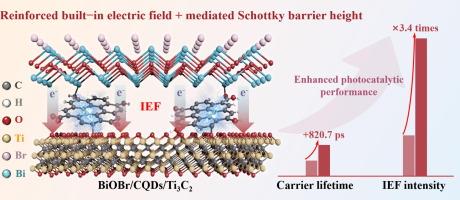
Reinforced built-in electric field and mediated Schottky barrier height via carbon quantum dots electronic bridges on BiOBr/Ti3C2 for efficient photocatalytic quinolone antibiotics degradation
Schottky junction-based photocatalysts formed by metal-semiconductor contact are attractive, but their photocatalytic performance is limited by poor built-in electric field (IEF) driving force and excessive Schottky barrier height (SBH). A prospective strategy for efficient charge transfer is modulating interface gap states by inserting viable buffer layer. Herein, carbon quantum dots (CQDs) were inserted in BiOBr/Ti3C2 Schottky heterojunction to form electronic bridges via Bi–O–C and Ti–O–C chemical bonds. The CQDs electronic bridges regulated charge spatial distribution, resulting in a 3.4-fold increased IEF, and facilitated efficient charge separation and transfer within BiOBr/CQDs/Ti3C2. The carrier lifetime of BiOBr/CQDs/Ti3C2 had been extended to 2357.8 ps, increasing effectively charge carrier density. Besides, the reinforced interfacial interaction by Ti–C–O and Bi–C–O bonding significantly reduced the SBH from 2.02 eV to 1.77 eV within BiOBr/CQDs/Ti3C2, accelerating charge transport across the metal-semiconductor interface. Remarkably, BiOBr/CQDs/Ti3C2 exhibited excellent photocatalytic degradation for multiple quinolone antibiotics (FQs), especially for Moxifloxacin (MOX, 96.1 %) within 120 min, which was 2.63, 2.48 and 1.84 times higher than that of BiOBr, BiOBr/CQDs and BiOBr/Ti3C2, respectively. Furthermore, the high environmental adaptability and recycle stability were revealed in BiOBr/CQDs/Ti3C2. This work provides a new strategy to construct electronic bridges in Schottky-based photocatalysts for enhancing photocatalytic activities
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


