具有四氢吡喃支架的螺环σ1 受体配体的合成与结构亲和力关系
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
σ1受体在调节人体的各种过程中发挥着关键作用;它参与神经退行性疾病和神经精神疾病的发展,并在多种人类肿瘤中过度表达,因此成为潜在候选药物的重要靶点。本项目合成了在 4 位和 9 位具有不同取代基的螺环 σ1 受体配体,并研究了它们对相关靶点的 σ1 受体亲和力和选择性。配体的 σ1 亲和力与它们的亲脂性(logD7.4 值)相关,从而了解了它们的亲脂配体效率(LLE)。(吡啶-3-基)甲基衍生物 5i 在高σ1 亲和力(Ki(σ1) = 3.9 nM)和选择性(250 倍)以及 5.8 的高 LLE 之间表现出良好的平衡。5i 具有较高的血浆蛋白结合率(89%),在小鼠肝脏微粒体和 NADPH 存在下具有良好的代谢稳定性(90 分钟后 83% 保持不变)。将螺环配体 5 的哌啶环的尺寸增大为氮杂环后,σ1 亲和力(Ki(5a) = 1.2 nM,Ki(23a) = 0.42 nM)和对σ2 受体的选择性(5a:45 倍,23a:150 倍)显著提高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
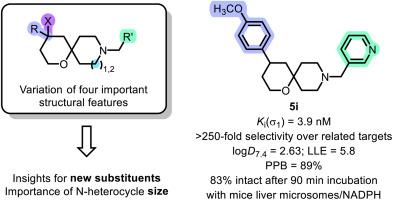
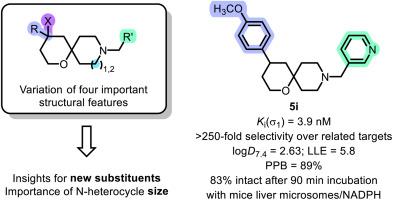
Synthesis and structure-affinity relationships of spirocyclic σ1 receptor ligands with tetrahydropyran scaffold
The σ1 receptor plays a key role in the regulation of various processes in the human body; it is involved in the development of neurodegenerative and neuropsychiatric diseases and is overexpressed in several human tumors rendering it an important target for potential drug candidates. In this project, spirocyclic σ1 receptor ligands with different substituents in 4- and 9-position were synthesized and investigated for their σ1 receptor affinity and selectivity over related targets. The σ1 affinity of the ligands was correlated with their lipophilicity (logD7.4 value) giving insight into their lipophilic ligand efficiency (LLE). The (pyridin-3-yl)methyl derivative 5i showed a promising balance of high σ1 affinity (Ki(σ1) = 3.9 nM) and selectivity (>250-fold) as well as high LLE of 5.8. 5i has a high plasma protein binding (89 %) and promising metabolic stability in the presence of mouse liver microsomes and NADPH (83 % intact after 90 min). Increasing the size of the piperidine ring of the spirocyclic ligands 5 to an azepane ring led to considerably increased σ1 affinity (Ki(5a) = 1.2 nM, Ki(23a) = 0.42 nM) and selectivity over σ2 receptors (5a: 45-fold, 23a: 150-fold).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


