用乙酰丙酮转化丰富的醛:揭示路易斯酸性盐在水中形成官能化呋喃的机理并改善对其的控制
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
通过多键形成反应扩展丰富的碳水化合物的反应探索和利用最近经历了一次复兴。此类反应可以在温和的温度下,从水中的可再生底物中形成类似药物的结构基团。本文采用反应跟踪和动力学建模相结合的方法,研究了在路易斯酸催化的加西亚-冈萨雷斯反应中,葡萄糖和其他简单碳水化合物与乙酰丙酮在水中转化为致密官能化呋喃的过程。利用实时 13C NMR 数据确定了呋喃产物的主要中间产物,动力学模型支持了推导出的途径并揭示了其能量学原理。尽管主要的二氢呋喃中间体是双环的,葡萄糖的 C4 氧连接到二氢呋喃环上,但使用 C4 功能化碳水化合物(麦芽糖)表明,该中间体并不是通向功能化呋喃的唯一途径中间体。使用几乎无溶剂的共晶混合物有利于分子间的初始步骤,并使葡萄糖和乙酰丙酮的转化在 323 K 温度下的 4 小时内基本完成,即使在只有 0.01 等量 Zr(IV)存在的情况下也是如此。由于转化是在最初的双分子反应和随后的单分子反应中进行的,足够高的底物浓度有利于最初的双分子反应生成多羟烷基呋喃,并与 C-糖基呋喃达到平衡。各种路易斯酸盐催化剂的使用表明,Zr(IV)催化对葡萄糖和乙酰丙酮的转化并不是唯一有利的。例如,在水中使用 Hf(IV)催化剂也能实现类似高效的乙酰丙酮烯醇化和反应。其他介质也被正确预测为可以在较温和的温度下沿着相同的途径催化反应,其中包括 ZnCl2 在水中的浓缩溶液,该溶液以前曾被描述为将多糖转化为脱水化学品的一种无毒且可回收的均相反应介质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
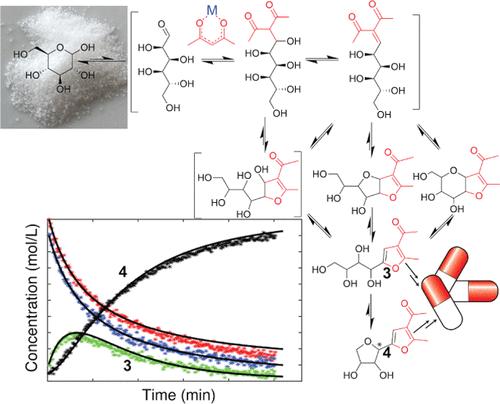
Conversion of Abundant Aldoses with Acetylacetone: Unveiling the Mechanism and Improving Control in the Formation of Functionalized Furans Using Lewis Acidic Salts in Water
The exploration and exploitation of reactions extending abundant carbohydrates by multiple-bond-forming reactions have recently experienced a renaissance. Such reactions can form drug-like structural motifs from renewable substrates in water at mild temperatures. A combined reaction tracking and kinetic modeling approach was conducted here for the conversion of glucose and other simple carbohydrates with acetylacetone to densely functionalized furans in the Lewis-acid-catalyzed Garcia Gonzalezreaction in water. Real-time 13C NMR data was used to identify major intermediates toward furan products, and kinetic modeling supports the deduced pathway and reveals its energetics. Albeit the main dihydrofuran intermediate is bicyclic, with the C4 oxygen of glucose attached to the dihydrofuran ring, the use of C4-functionalized carbohydrate (maltose) shows that this intermediate is not the only on-pathway intermediate toward functionalized furans. The use of nearly solvent-free eutectic mixtures favors the intermolecular initial steps and renders the conversion of glucose and acetylacetone largely complete within 4 h at 323 K, even in the presence of only 0.01 equiv Zr(IV). As the conversion proceeds in an initial bimolecular and subsequent unimolecular reaction, sufficiently high substrate concentrations favor the initial bimolecular reaction to polyhydroxyalkyl furan, which equilibrates with C-glycosyl furan. The use of various Lewis acid salt catalysts indicates that the conversion of glucose and acetylacetone is not uniquely favored by Zr(IV) catalysis. For instance, similarly efficient enolization of acetylacetone and reaction are achieved using Hf(IV) catalysis in water. Alternative media were correctly predicted to catalyze the reaction at milder temperatures along the same pathway, including a concentrated solution of ZnCl2 in water that had previously been described as a nontoxic and recyclable homogeneous reaction medium for converting polysaccharides into dehydrated chemicals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


