双鱼藤酸抑制饮食诱导的肝脂肪变性,与 ATP 柠檬酸裂解酶无关
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
ATP 柠檬酸裂解酶(ACLY)合成乙酰-CoA,用于新生脂肪生成(DNL),在代谢功能障碍相关性脂肪肝中,DNL 会升高。降低低密度脂蛋白胆固醇的药物贝门冬氨酸(BPA)可抑制肝脏乙酰胆碱转化酶(ACLY),这也会改善小鼠的脂肪变性。虽然 BPA 能有效抑制肝脏 DNL 并增加脂肪分解,但尚不清楚 ACLY 是否是其降低肝脏甘油三酯的主要分子靶点。我们的研究表明,在西式饮食中,肝脏 ACLY 单独或与乙酰-CoA 合成酶 ACSS2 一起缺失会意外加剧脂肪变性,这与 PPARα 靶基因表达和脂肪酸氧化减少有关。重要的是,在 WT 小鼠和肝脏 ACLY 基因敲除小鼠中,双酚 A 处理可改善西方饮食介导的三酰甘油积累,这表明双酚 A 对肝脏脂肪变性的主要影响与 ACLY 无关。这些数据共同表明,肝脏 ACLY 在抑制饮食依赖性脂质积累方面发挥了意想不到的作用,而且双酚 A 对肝脏脂质代谢产生的实质性影响与 ACLY 无关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
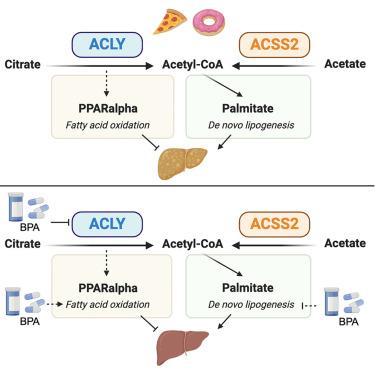
Bempedoic acid suppresses diet-induced hepatic steatosis independently of ATP-citrate lyase
ATP citrate lyase (ACLY) synthesizes acetyl-CoA for de novo lipogenesis (DNL), which is elevated in metabolic dysfunction-associated steatotic liver disease. Hepatic ACLY is inhibited by the LDL-cholesterol-lowering drug bempedoic acid (BPA), which also improves steatosis in mice. While BPA potently suppresses hepatic DNL and increases fat catabolism, it is unclear if ACLY is its primary molecular target in reducing liver triglyceride. We show that on a Western diet, loss of hepatic ACLY alone or together with the acetyl-CoA synthetase ACSS2 unexpectedly exacerbates steatosis, linked to reduced PPARα target gene expression and fatty acid oxidation. Importantly, BPA treatment ameliorates Western diet-mediated triacylglyceride accumulation in both WT and liver ACLY knockout mice, indicating that its primary effects on hepatic steatosis are ACLY independent. Together, these data indicate that hepatic ACLY plays an unexpected role in restraining diet-dependent lipid accumulation and that BPA exerts substantial effects on hepatic lipid metabolism independently of ACLY.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


