靶向 BCAT1 介导的 BCAA 代谢的小分子抑制 SHOC2-RAS-ERK 的激活,从而诱导三阴性乳腺癌细胞凋亡
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
导言三阴性乳腺癌(TNBC)是乳腺癌中恶性程度最高、预后最差的亚型。探索新的致癌因素和 TNBC 的治疗药物仍是改善预后的重点。支链氨基酸转氨酶1(BCAT1)是支链氨基酸(BCAA)代谢过程中的一个关键酶,它与多种肿瘤的发生发展有关,但其在TNBC中的致癌功能和机制仍不清楚。本研究旨在通过探索EB抑制TNBC的靶点和药理机制,发现TNBC的新治疗靶点和潜在抑制剂,并阐明TNBC的新致病机制。方法采用小鼠模型和细胞表型实验研究EB对TNBC的抑制作用。方法利用小鼠模型和细胞表型实验研究了EB对TNBC的抑制作用,并利用基于活性的蛋白质分析(ABPP)技术、pull down-WB、CETSA-WB和MST发现和验证了EB的靶点。通过临床数据分析和生化实验确定了 BCAT1 的致癌作用。为了阐明EB抑制TNBC的机制,研究人员采用了多种方法,包括但不限于高效液相色谱法和蛋白质组测序法。我们确定 BCAT1 是 EB 的直接靶点,并证实 BCAT1 对 TNBC 的发展至关重要。EB抑制了BCAT1参与的BCAA代谢,减少了BCAA(包括Leu、Ile和Val)的合成,从而抑制了SHOC2(一种富含Leu的重复蛋白)的表达和下游SHOC2参与的RAS-ERK信号通路,最终导致TNBC细胞凋亡。结论总之,这项研究不仅阐明了 BCAT1 及其下游 SHOC2-RAS-ERK 信号轴在 TNBC 进展中的致癌作用,还为针对 BCAT1 或 BCAA 代谢的潜在疗法(单独使用 EB 或与其抑制剂坎地沙坦联合使用)治疗 TNBC 开辟了途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
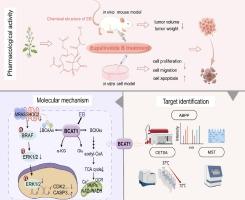
Small-molecule targeting BCAT1-mediated BCAA metabolism inhibits the activation of SHOC2-RAS-ERK to induce apoptosis of Triple-negative breast cancer cells
Introduction
Triple-negative breast cancer (TNBC) is the most malignant subtype of breast cancer with the worst prognosis. Exploring novel carcinogenic factors and therapeutic drugs for TNBC remains a focus to improve prognosis. Branched-chain amino acid transaminase 1 (BCAT1), a crucial enzyme in branched-chain amino acid (BCAA) metabolism, has been linked to various tumor developments, but its carcinogenic function and mechanism in TNBC remain unclear. Eupalinolide B (EB) is a naturally-derived small-molecule with anti-tumor activity, but its role in TNBC remains unknown.Objectives
By exploring the targets and pharmacological mechanisms of EB in inhibiting TNBC, this study aimed to discover novel therapeutic targets and potential inhibitors for TNBC, and elucidate novel pathogenic mechanisms of TNBC.Methods
The inhibitory effect of EB on TNBC was investigated using mouse models and cellular phenotypic experiments. Activity-based protein profiling (ABPP) technology, pull down-WB, CETSA-WB and MST were utilized to discover and validate the targets of EB. The oncogenic role of BCAT1 was determined through clinical data analysis and biochemical experiments. To elucidate the mechanism by which EB inhibited TNBC, many methods, including but not limited to HPLC and proteomic sequencing were used.Results
We found that EB significantly inhibited TNBC progression. We identified BCAT1 as the direct target of EB and confirmed that BCAT1 was critical for TNBC development. EB inhibited BCAT1-involved BCAA metabolism to reduce the synthesis of BCAAs (including Leu, Ile, and Val), thereby inhibiting SHOC2 (a Leu-rich repeat protein) expression and the downstream SHOC2-participating RAS-ERK signaling pathway, ultimately leading to apoptosis of TNBC cells.Conclusion
Collectively, this study not only elucidates the oncogenic role of BCAT1 and its downstream SHOC2-RAS-ERK signaling axis in TNBC progression but also opens up avenues for potential therapies targeting BCAT1 or BCAA metabolism (using EB alone or in combination with its inhibitor candesartan) for TNBC treatment.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


