区分氧进化反应中的 OH- 氧化和 H2O 氧化
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
氧进化反应(OER)中的 OH-/H2O 反应物判别是一个关键问题,但却没有得到很好的解决。这导致了不合理的活性比较、对 OER 机理的误解以及理论计算模型的不统一,而不考虑 OH- 和 H2O 氧化之间的热力学/动力学差异。在这里,我们通过调整界面 OH- 浓度来区分 OH- 和 H2O 氧化。结合 OER 动力学分析和基于同位素标记的原位 16OH-/H218O 差分电化学质谱法,我们研究了 OH- 氧化和 H2O 氧化各自的电化学氧化行为。我们发现,相对于 H2O 氧化,OH- 氧化的起始电位要低∼550 mV,而且塔菲尔图给出的 OH- 氧化斜率为∼50 mV dec-1,大大低于模型 CoOOH 催化剂上 H2O 氧化的斜率∼200 mV dec-1。这项工作要求将区分 OH-/H2O 氧化作为未来 OER 活性评估和机理研究的先决条件。本文章由计算机程序翻译,如有差异,请以英文原文为准。
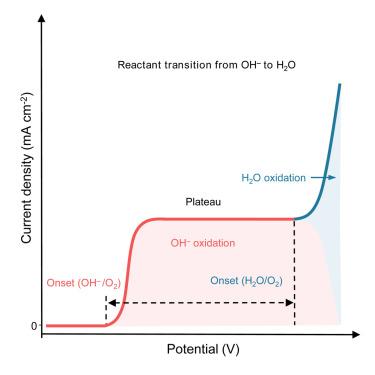
Discrimination between OH− and H2O oxidation for oxygen evolution reaction
OH−/H2O-reactant discrimination for the oxygen evolution reaction (OER) is a critical but not well resolved issue. This has led to unreasonable activity comparisons, misinterpreted OER mechanisms, and ununified models for theoretical calculations regardless of the thermodynamic/kinetic difference between OH− and H2O oxidation. Here, we discriminate between OH− and H2O oxidation by tuning the interfacial OH− concentration. Combining OER kinetic analysis with in situ 16OH−/H218O isotopic labeling-based differential electrochemical mass spectrometry, we examine the respective electrochemical oxidation behaviors between OH− and H2O oxidation. We find that OH− oxidation presents ∼550 mV lower onset potential relative to H2O oxidation and that Tafel plotting gives slopes of ∼50 mV dec−1 for OH− oxidation, which is substantially lower than those of ∼200 mV dec−1 for H2O oxidation on a model CoOOH catalyst. This work calls for the discrimination of OH−/H2O oxidation as the prerequisite for future OER activity evaluation and mechanism studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


