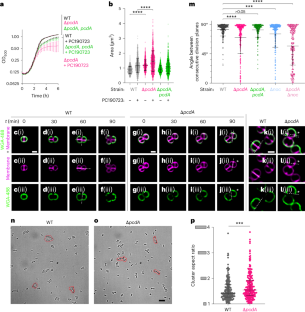
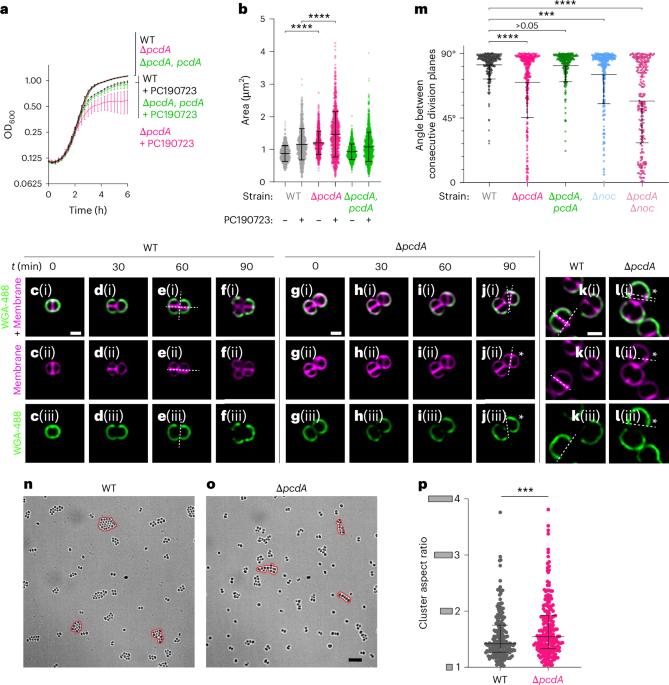
PcdA 促进金黄色葡萄球菌的正交分裂面选择
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
细菌病原体金黄色葡萄球菌通过在两个交替的正交平面上分裂生长。这些细胞分裂平面如何正确定位尚不清楚。在这里,我们利用化学遗传筛选方法确定了 PcdA 作为分裂平面定位因子。分子生物学和成像方法揭示了 pcdA 突变细菌的非正交分裂平面选择。PcdA 是 McrB AAA+ NTPase 家族的一个结构和功能改变的成员,通常作为限制酶亚基存在。PcdA 与管蛋白样分裂体成分 FtsZ 和结构蛋白 DivIVA 相互作用;它还定位到未来的细胞分裂位点。PcdA 的多聚化、定位和功能都依赖于 NTPase 的活性。我们认为,DivIVA/PcdA 复合物能吸引未聚合的 FtsZ 沿正确的细胞分裂平面聚集。虽然 pcdA 缺失并不影响金黄色葡萄球菌在多种实验室条件下的生长,但它的集群生长模式被破坏,对细胞壁靶向抗生素的敏感性增加,对小鼠的毒力降低。我们认为,金黄色葡萄球菌在正交分裂平面上交替分裂所产生的特征性集群生长模式可能会保护该细菌免受宿主防御的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
PcdA promotes orthogonal division plane selection in Staphylococcus aureus
The bacterial pathogen, Staphylococcus aureus, grows by dividing in two alternating orthogonal planes. How these cell division planes are positioned correctly is not known. Here we used chemical genetic screening to identify PcdA as a division plane placement factor. Molecular biology and imaging approaches revealed non-orthogonal division plane selection for pcdA mutant bacteria. PcdA is a structurally and functionally altered member of the McrB AAA+ NTPase family, which are often found as restriction enzyme subunits. PcdA interacts with the tubulin-like divisome component, FtsZ, and the structural protein, DivIVA; it also localizes to future cell division sites. PcdA multimerization, localization and function are NTPase activity-dependent. We propose that the DivIVA/PcdA complex recruits unpolymerized FtsZ to assemble along the proper cell division plane. Although pcdA deletion did not affect S. aureus growth in several laboratory conditions, its clustered growth pattern was disrupted, sensitivity to cell-wall-targeting antibiotics increased and virulence in mice decreased. We propose that the characteristic clustered growth pattern of S. aureus, which emerges from dividing in alternating orthogonal division planes, might protect the bacterium from host defences. PcdA interacts with DivIVA and FtsZ, promoting Z-ring formation and division plane selection in Staphylococcus aureus, which increases virulence in mice and reduces sensitivity to cell-wall-targeting antibiotics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


