用于稳定合成碱土金属过氧化物的自清洁电极
IF 34.9
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
碱土金属过氧化物(MO2,M = Ca、Sr、Ba)是一类多功能、清洁的固体氧化剂,而合成过程通常会消耗过量的过氧化氢(H2O2)。在这里,我们发现通过电极表面的双电子电化学氧还原(2e- ORR)合成的 H2O2 可以在碱性环境中高效、持久地消耗,从而生产出高纯度的 MO2。关键因素在于原位生成的 MO2 能及时从自清洁电极上脱离,固体产物自发地从电极上脱离,从而解决了堵塞问题。自清洁电极是通过构建高活性催化剂的微/纳米结构并进行适当的表面修饰实现的。在实验中,MO2 的电化学合成实现了前所未有的累积选择性(约 99%)和持久性(1,000 h,50 mA cm-2)。此外,还验证了 CaO2 和 H2O2 在水动力空化作用下降解四环素的相似效率(H2O2 和 CaO2 的降解效率分别为 87.9% 和 93.6%)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
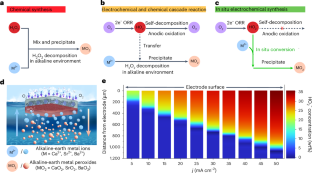

Self-cleaning electrode for stable synthesis of alkaline-earth metal peroxides
Alkaline-earth metal peroxides (MO2, M = Ca, Sr, Ba) represent a category of versatile and clean solid oxidizers, while the synthesis process usually consumes excessive hydrogen peroxide (H2O2). Here we discover that H2O2 synthesized via two-electron electrochemical oxygen reduction (2e− ORR) on the electrode surface can be efficiently and durably consumed to produce high-purity MO2 in an alkaline environment. The crucial factor lies in the in-time detachment of in situ-generated MO2 from the self-cleaning electrode, where the solid products spontaneously detach from the electrode to solve the block issue. The self-cleaning electrode is achieved by constructing micro-/nanostructure of a highly active catalyst with appropriate surface modification. In experiments, an unprecedented accumulated selectivity (~99%) and durability (>1,000 h, 50 mA cm−2) are achieved for electrochemical synthesis of MO2. Moreover, the comparability of CaO2 and H2O2 for tetracycline degradation with hydrodynamic cavitation is validated in terms of their close efficacies (degradation efficiency of 87.9% and 93.6% for H2O2 and CaO2, respectively). This work introduces a reaction system that enables the in situ conversion of H2O2 generated by 2e− ORR at the self-cleaning electrode surface into alkaline-earth metal peroxides. The solid oxidizer CaO2 exhibits comparable efficiency to H2O2 for tetracycline degradation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


